Abstract
Mini Review
Targeted and non-targeted effects of radiation in mammalian cells: An overview
Rita Ghosh* and Surajit Hansda
Published: 12 April, 2021 | Volume 5 - Issue 1 | Pages: 013-019
Radiation of different wavelengths can kill living organisms, although, the mechanism of interactions differs depending on their energies. Understanding the interaction of radiation with living cells is important to assess their harmful effects and also to identify their therapeutic potential. Temporally, this interaction can be broadly divided in three stages – physical, chemical and biological. While radiation can affect all the important macromolecules of the cells, particularly important is the damage to its genetic material, the DNA. The consequences of irradiation include- DNA damage, mutation, cross-linkages with other molecules, chromosomal aberrations and DNA repair leading to altered gene expression and/or cell death. Mutations in DNA can lead to heritable changes and is important for the induction of cancer. While some of these effects are through direct interaction of radiation with the target, radiation can interact with the surrounding environment to result in its indirect actions. The effects of radiation depend not only on the total dose but also on the dose rate, LET etc. and also on the cell types. However, action of radiation on organisms is not restricted to interactions with irradiated cells, i.e. target cells alone; it also exerts non-targeted effects on neighboring unexposed cells to produce productive responses; this is known as bystander effect. The bystander effects of ionizing radiations are well documented and contribute largely to the relapse of cancer and secondary tumors after radiotherapy. Irradiation of cells with non-ionizing Ultra-Violet light also exhibits bystander responses, but such responses are very distinct from that produced by ionizing radiations.
Read Full Article HTML DOI: 10.29328/journal.abb.1001023 Cite this Article Read Full Article PDF
Keywords:
Ionizing radiation; Non-ionizing radiation; Targeted action; non-targeted action; bystander effect
References
- Assmus A, Early History of X–rays, Summer. 1995: 10-24.
- Shepherd JA, Ng BK, Fan B, Schwartz AV, Cawthon P, et al. Modeling the shape and composition of the human body using dual energy X-ray absorptiometry images. PloS One. 2017; 12: e0175857. PubMed: https://pubmed.ncbi.nlm.nih.gov/28423041/
- Greenwald HP. Who survives cancer? Univ of California Press. 1992.
- Lea DE. Actions of radiations on living cells. Actions of radiations on living cells. 1955.
- Manna D, Ghosh R. Effect of radiofrequency radiation in cultured mammalian cells: A review. Electromagn Biol Med. 2016; 35: 265-301. PubMed: https://pubmed.ncbi.nlm.nih.gov/27053138/
- Hansda S, Mitra A, Ghosh R. Studies to explore the UVA photosensitizing action of 9-phenylacridine in cells by interaction with DNA. Nucleosides Nucleotides Nucleic Acids. 2021; 1-30. PubMed: https://pubmed.ncbi.nlm.nih.gov/33586599/
- Seltzer SM, Bartlett DT, Burns DT, Dietze G, Menzel HG, et al. Fundamental quantities and units for ionizing radiation. ICRU Journal. 2011; 11: 1.
- Valentin J. Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (wR): ICRP Publication 92. Ann ICRP. 2003; 33: 1-21.
- Alpen EL. Radiation biophysics. Academic press; 1997.
- Samuel AH, Magee JL. Theory of radiation chemistry. II. Track effects in radiolysis of water. J Chem Phys. 1953; 21: 1080-1087.
- Harrison FL, Anderson SL. Taxonomic and developmental aspects of radiosensitivity. Lawrence Livermore National Lab., CA (United States). 1996.
- Pfafflin JR, Ziegler EN, editors. Encyclopedia of environmental science and engineering. Taylor & Francis; 1(A-L) fifth edition. 1992.
- Hall EJ, Giaccia AJ. Molecular mechanisms of DNA and chromosome damage and repair. Radiobiology for the radiologist. 7th ed. Philadelphia: Lippincott Williams & Wilkins. 2012: 12-34.
- Friedberg EC, Walker GC, Siede W, Putte PV. DNA repair and mutagenesis. Trends in Biochemical Sciences. 1995; 20: 440.
- Goodarzi AA, Anikin A, Pearson DD. Environmental sources of ionizing radiation and their health consequences. Genome Stability, Academic Press. 2016; 569-581.
- Hack, RC. Ionizing radiation. Occupational Health Practice. Butterworth-Heinemann, 1989. 151-174.
- Cannan WJ, Pederson DS. Mechanisms and consequences of double‐strand DNA break formation in chromatin. J Cell Physiol. 2016; 231: 3-14. PubMed: https://pubmed.ncbi.nlm.nih.gov/26040249/
- Leadon, Steven A. Repair of DNA damage produced by ionizing radiation: A mini review. Semin Radiat Oncol. 1996; 6: 295–305. PubMed: https://pubmed.ncbi.nlm.nih.gov/10717187/
- Little JB. Principal Cellular and Tissue Effects of Radiation. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker. 2003. PubMed: https://www.ncbi.nlm.nih.gov/books/NBK12344/
- Li M, You L, Xue J, Lu Y. Ionizing radiation-induced cellular senescence in normal, non-transformed cells and the involved DNA damage response: A mini review. Front Pharmacol. 2018; 9: 522. PubMed: https://pubmed.ncbi.nlm.nih.gov/29872395/
- Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014; 21: 260–292. PubMed: https://pubmed.ncbi.nlm.nih.gov/24382094/
- Little JB. Cellular effects of ionizing radiation. New Engl J Med. 1968; 278: 369-376. PubMed: https://pubmed.ncbi.nlm.nih.gov/4865594/
- Zhang H, Koch CJ, Wallen CA, Wheeler KT. Radiation-induced DNA damage in tumors and normal tissues. III. Oxygen dependence of the formation of strand breaks and DNA-protein crosslinks. Radiat Res. 1995; 142: 163-168. PubMed: https://pubmed.ncbi.nlm.nih.gov/7724730/
- Alper T, Bryant PE. Reduction in oxygen enhancement ratio with increase in LET: Tests of two hypotheses. Int J Radiat Biol Relat Stud Phys Chem Med. 1974; 26: 203-218. PubMed: https://pubmed.ncbi.nlm.nih.gov/4609938/
- Barendsen GW. Parameters of linear-quadratic radiation dose-effect relationships: dependence on LET and mechanisms of reproductive cell death. Int J Radiat Bio. 1997; 71: 649-655. PubMed: https://pubmed.ncbi.nlm.nih.gov/9246179/
- Barendsen GW. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Bio Phys. 1982; 8: 1981-1997. PubMed: https://pubmed.ncbi.nlm.nih.gov/6759484/
- Joksic G, Petrovic S, Ilic Z. Age-related changes in radiation-induced micronuclei among healthy adults. Braz J Med Bio Res. 2004; 37: 1111-1117. PubMed: https://pubmed.ncbi.nlm.nih.gov/15273813/
- Bushong SC. Radiologic Science for Technologists-E-Book: Physics. Biology, and Protection: Elsevier Health Sciences. 2013.
- Valentin J. The 2007 recommendations of the international commission on radiological protection. Elsevier; 2008.
- Cardis E, Gilbert ES, Carpenter L, Howe G, Kato I, et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res. 1995; 142: 117-132. PubMed: https://pubmed.ncbi.nlm.nih.gov/7724726/
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003; 159: 567-580.
- Baskar R. Emerging role of radiation induced bystander effects: Cell communications and carcinogenesis. Genome integr. 2010; 1: 13.
- Grossweiner LI, Smith KC. The science of photobiology. Photophysics. 2nd Edition. 1989: 1-46.
- Kiefer J. Effects of ultraviolet radiation on DNA. In Chromosomal Alterations. Springer, Berlin, Heidelberg. 2007: 39-53.
- Santos AL, Moreirinha C, Lopes D, Esteves AC, Henriques I, et al. Effects of UV radiation on the lipids and proteins of bacteria studied by mid-infrared spectroscopy. Environ Sci Technol. 2013; 47: 6306-6315. PubMed: https://pubmed.ncbi.nlm.nih.gov/23692317/
- World Health Organization. Health and environmental effects of ultraviolet radiation: a summary of Environmental health criteria 160, ultraviolet radiation. World Health Organization. 1995.
- Widel M. Bystander effect induced by UV radiation; why should we be interested? Postepy Hig Med Dosw (Online). 2012; 66: 828-837. PubMed: https://pubmed.ncbi.nlm.nih.gov/23175338/
- Ghosh R, Guha D, Bhowmik S. UV released factors induce antioxidant defense in A375 cells. Photochem Photobiol. 2012; 88: 708-716. PubMed: https://pubmed.ncbi.nlm.nih.gov/22296560/
- Ghosh R, Bhaumik G. Supernatant medium from UV-irradiated cells influences the cytotoxicity and mutagenicity of V79 cells. Mutation Research/Environmental Mutagenesis and Related Subjects. 1995; 335: 129-135. PubMed: https://pubmed.ncbi.nlm.nih.gov/7477043/
- Ghosh R, Guha D, Bhowmik S, Karmakar S. Antioxidant enzymes and the mechanism of the bystander effect induced by ultraviolet C irradiation of A375 human melanoma cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2013; 757: 83-90. PubMed: https://pubmed.ncbi.nlm.nih.gov/23845763/
- Ghosh R, Guha D, Bhowmik S, Karmakar S. Some UV-bystander effects are mediated through induction of antioxidant defense in mammalian cells. Ind J Biochem Biophys. 2012; 49: 371-378. PubMed: https://pubmed.ncbi.nlm.nih.gov/23259324/
- Guha D, Bhowmik S, Ghosh R. Influence of ultraviolet C bystander effect on inflammatory response in A375 cells subsequent exposure to ultraviolet C and hydrogen peroxide. Ind J Biochem Biophys. 2014; 51: 552-558. PubMed: https://pubmed.ncbi.nlm.nih.gov/25823229/
- Hua H, Cheng J, Bu W, Liu J, Ma W, et al. 5-Aminolevulinic Acid-Based Photodynamic therapy Pretreatment Mitigates Ultraviolet A-Induced Oxidative Photodamage. Oxid Med Cell Longev. 2018; 2018: 9420745. PubMed: https://pubmed.ncbi.nlm.nih.gov/30524664/
- Hansda S, Ghosh G, Ghosh R. 9-phenyl acridine photosensitizes A375 cells to UVA radiation. Heliyon. 2020; 6: e04733. PubMed: https://pubmed.ncbi.nlm.nih.gov/32944667/
- Ghosh R. Role of Proteases in Photo-aging of the Skin. In Proteases in Physiology and Pathology 2017; 435-449.
Figures:
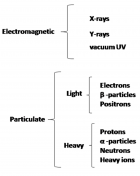
Figure 1
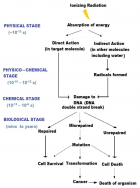
Figure 2
Similar Articles
-
Targeted and non-targeted effects of radiation in mammalian cells: An overviewRita Ghosh*,Surajit Hansda. Targeted and non-targeted effects of radiation in mammalian cells: An overview. . 2021 doi: 10.29328/journal.abb.1001023; 5: 013-019
Recently Viewed
-
Adult Neurogenesis: A Review of Current Perspectives and Implications for Neuroscience ResearchAlex, Gideon S*,Olanrewaju Oluwaseun Oke,Joy Wilberforce Ekokojde,Tolulope Judah Gbayisomore,Martina C. Anene-Ogbe,Farounbi Glory,Joshua Ayodele Yusuf. Adult Neurogenesis: A Review of Current Perspectives and Implications for Neuroscience Research. J Neurosci Neurol Disord. 2024: doi: 10.29328/journal.jnnd.1001102; 8: 106-114
-
Late discover of a traumatic cardiac injury: Case reportBenlafqih C,Bouhdadi H*,Bakkali A,Rhissassi J,Sayah R,Laaroussi M. Late discover of a traumatic cardiac injury: Case report. J Cardiol Cardiovasc Med. 2019: doi: 10.29328/journal.jccm.1001048; 4: 100-102
-
A two-phase sonographic study among women with infertility who first had normal sonographic findingsKalu Ochie*,Abraham John C. A two-phase sonographic study among women with infertility who first had normal sonographic findings. Clin J Obstet Gynecol. 2022: doi: 10.29328/journal.cjog.1001117; 5: 101-103
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024: doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Analysis of Psychological and Physiological Responses to Snoezelen Multisensory StimulationLucia Ludvigh Cintulova,Jerzy Rottermund,Zuzana Budayova. Analysis of Psychological and Physiological Responses to Snoezelen Multisensory Stimulation. J Neurosci Neurol Disord. 2024: doi: 10.29328/journal.jnnd.1001103; 8: 115-125
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."