Mini Review
ABC and MFS Transporters: A reason for Antifungal drug resistance

Neelabh1* and Karuna Singh1,2
1Department of Zoology (MMV), Institute of Science, Banaras Hindu University, Varanasi -221005, Uttar Pradesh, India
2Mentor, Department of Zoology (MMV), Institute of Science, Banaras Hindu University, Varanasi -221005, Uttar Pradesh, India
*Address for Correspondence: Neelabh, Department of Zoology (MMV), Institute of Science, Banaras Hindu University, Varanasi -221005, Uttar Pradesh, India, Email: [email protected]
Dates: Submitted: 23 December 2017; Approved: 04 January 2018; Published: 05 January 2018
How to cite this article: Neelabh, Singh K. ABC and MFS Transporters: A reason for Antifungal drug resistance. Arch Biotechnol Biomed. 2018; 2: 001-007. DOI: 10.29328/journal.abb.1001009
Copyright License: © 2018 Neelabh, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Fungi; Resistance; Transporters; ABC; MFS
Abstract
Fungi cause a variety of diseases and are difficult to treat owing to their eukaryotic nature resulting in dearth of antifungal targets at hand. This problem is further elevated many folds due to the resistance mechanisms of fungi through which they circumvent the antifungal drugs administered for therapeutic purposes. Fungi have a variety of strategies for obtaining these resistances, amongst them pivotal role is played by the ABC and MFS transporters. This article encompasses the important genes and their respective roles of both the classes of the transporters in different species of fungi.
Introduction
Our natural environment encompasses a humongous number of micro-organisms such as bacteria, fungi, viruses etc, having varied habitats and an intrinsic capacity to adapt to it. These micro-organisms very often are associated with the mortality and morbidity of the human beings. Amongst these micro-organisms are fungi which are ubiquitous in environment and are saprophytic or parasitic in nature. The impact of fungi on human health can be accessed by the data saying that invasive fungal infections IFIs are responsible for around 1.5 million deaths worldwide each year [1-3]. They being eukaryotic in nature are harder to kill as the targets unique to them are fewer in number. Apart from this they have an added advantage to undergo genetic modifications thus making them less susceptible and resistant to the ongoing therapeutics. The development of resistance in fungi is a major scientific challenge that has to be addressed by the scientific community and the pharmaceutical industry unanimously. Some major antifungal drugs and the fungal species having developed resistance against them have been depicted in table 1 [4].
| Table 1: | |
| Drugs | Resistant fungal species |
| Fluconazole | Candida albicans, C. glabrata, C. tropicalis, C. krusei, C. dubliniensis, Cryptococcus neoformans, Histoplasma capsulatum, Aspergillus fumigatus, A. flavus |
| Amphotericin B | C. albicans, C. lusitaniae, C. neoformans |
| Flucytosine | C. albicans, C. neoformans, Aspergillus spp. |
| Terbinafine | C. albicans |
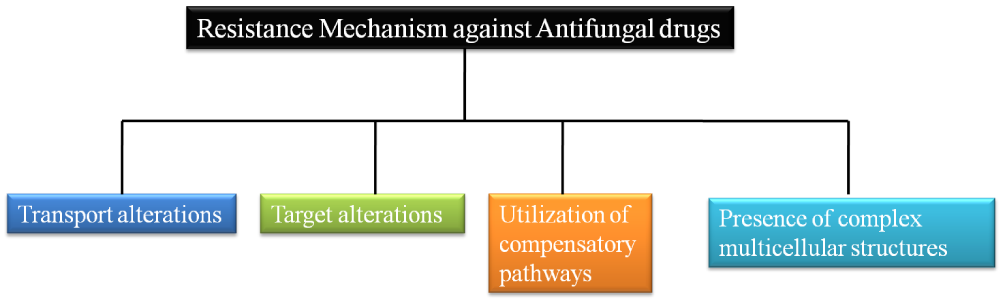
Chiefly, there are four strategies that are exploited by the fungi so as to confer antifungal resistance (Figure 1). However, this short article is aimed to identify the genes and their roles in conferring the antifungal resistance by means of transport alterations through ABC and MFS transporters [5].
Figure 1: Figure showing the strategies of resistance mechanism employed by fungi to confer antifungal resistance.
Transport alterations
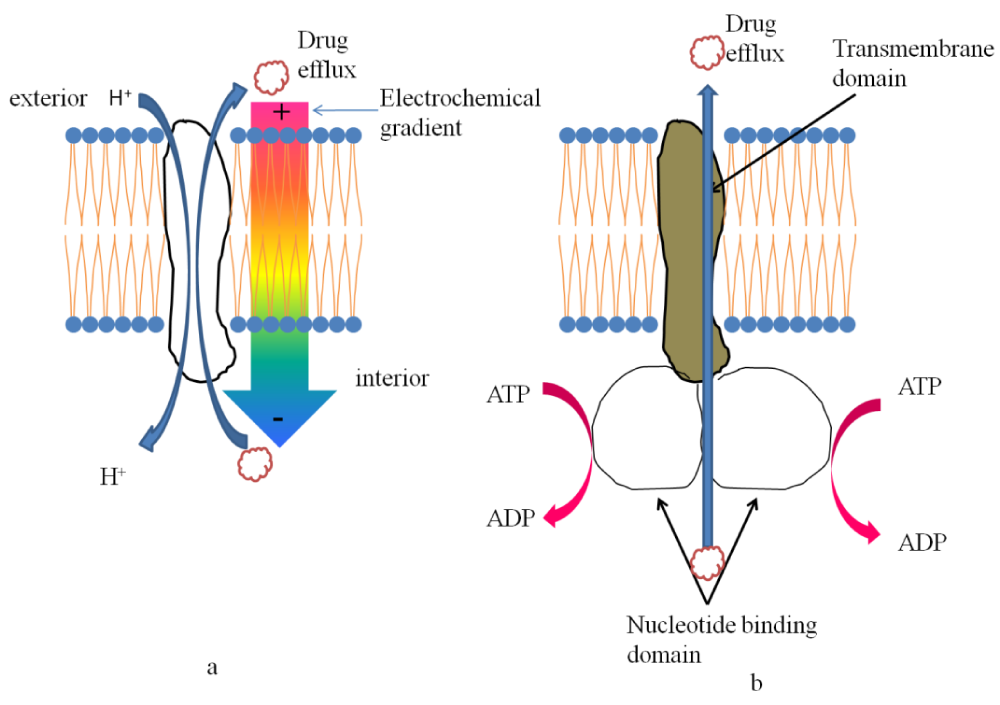
Survival of a microorganism in a natural environment is dependent upon two factors a) its ability to secrete toxic compounds against the competing microorganisms and b) its ability to survive any such attacks. Antifungal drugs administered create a hostile environment for the fungi and in order to counter it fungi use their transporters viz. ATP-binding cassette (ABC) and the major facilitator superfamily (MFS) transporters [5]. A diagrammatic representation of both of them has been provided in figure 2.
ABC Transporters: ABC transporters, located not only in the plasma membrane but also in the membrane of the organelles, comprise of two transmembrane domains (TMD) and two cytoplasmic nucleotide binding domain (NBD). TMDs function as transmembrane helices facilitating the transport whereas NBDs provides site for ATP hydrolysis [6]. The precise arrangement of the NBDs and TMDs inside a pump polypeptide (transporter) depends on the type of the pump itself. There are three main subfamilies of ABC transporters based on the classification made in Saccharomyces cerevisiae, viz. the pleiotropic drug resistance (PDR), multi-drug resistance (MDR), and multidrug resistance-associated protein (MRP) (cf. human CFTR) subfamilies [7-9]. Amongst these the most important fungal subfamily often associated with the fungal drug resistance is the PDR family, the prime example being Pdr5p in S. cerevisiae [10-12].
In case of Candida species, the over expression of Candida drug resistance 1 (CDR 1) and Candida drug resistance 2 (CDR 2) genes are responsible for the induction of azole resistance, CDR1 being a major contributor [13-18]. The function of CDR1 is not restricted to azole resistivity but also works as a multidrug resistance (MDR) gene. Further research on C. albicans has shown that CaCDR3 and CaCDR4 function as phospholipid flippases. CaCdr3p although shows high sequence conservation with CaCdr1p and CaCdr2p but does not contribute to resistance against flucytosine [19]. Similarly, no involvement of CaCDR4 gene was found in flucytosine resistance [20]. Homology based studies suggest that orthologs of SNQ2 and YOR1 are involved in providing resistance against 4-nitro-1-oxido-quinoline [21] and aureobasidin A, respectively [22].
Thirty percent of clinical isolates of Candida glabrata portray a moderate innate resistance to azoles. It has also been observed that this innate resistance can mount up during the course of treatment or prophylaxix with azole drugs especially fluconazole which persists even after the treatment culminates [23]. A noteworthy point that needs to be mentioned here is that due to the extensive use of fluconazole the cross resistance in C. glabrata against other azoles (itraconazole, ketoconazole or voriconazole) has increased [24]. There are about 18 ABC transporters that have been identified in C.glabrata. Amongst them, CgCDR1, CgCDR2, CgSNQ2 are the functional analogues of CDR1 and CDR2. Over expression of CgCDR1 has been found to confer resistance against azoles [25,26]. This efflux pump utilizes ATP phosphorylation as an energy source and apart from azoles has a wide range of structurally and functionally diverse group of compounds as substrates. Further CgAUS1, has been identified which is responsible for the rejuvenation of the lost ergosterol due to the activity of azoles [27]. Unlike other species of Candida, C. krusei is found to be resistant to fluconazole, the reason being the reduced susceptibility of the drug target Erg11p to azole antifungals, whereas a majority of the strains are found to be susceptible in a dose-dependent manner to itraconazole [28-30]. Studies based on the degenerate primers have helped us to identify CkABC1 and CkABC2 as the only known ABC transporters till date in C.krusei. Amongst CkABC1 and CkABC2 the latter was found strongly induced in response to azoles whereas the former was minimally expressed under all growth conditions tested.
Studies based on identification of functional analogues of CDR1 and CDR2 in C. dubliniensis resulted in CdCDR1 and CdCDR2 in C. krusei, ABC1 and ABC2 in C. tropicalis, CDR1 homologue and in Cryptococcus neoformans, CnAFR1 also function as important genes for azole resistance [5,31-33]. In Aspergillus fumigatus the ABC transporter genes responsible for imparting resistance are atrF and atrI. It has been found that atrI mediates resistance to itraconazole and voriconazole whereas atrI mediated resistance only to voriconazole in case of S.cerevisiae [34].
A. fumigatus genome comprises of 49 ABC transporters, however there is not much evidence available to link any ABC transporter with clinically relevant antifungal drug resistance. The probable reason behind this is that the specificity of all the transporters is limited henceforth we can conclude that efflux in this case is not related to imparting resistance against antifungal drugs [35]. Multiple copies of the genes encoding several enzymes participating in the ergosterol bisosynthesis pathway have been found in the genome of Aspergillus species example CYP 51 gene. Gene knockout studies have depicted that Cyp51Ap (AfuCyp51Ap) is responsible for the innate resistance to fluconazole and ketoconazole [36]. Further, the ABC transporters that have been isolated and characterized are AtrA, AtrB, AtrC, AtrC2, and AtrD [37-40]. AtrA and AtrB are PDR ABC pumps whereas AtrC, AtrC2 and AtrD serve as MDR class of transporters.
In case of Cryptococcus species, 54 ABC transporters have been reported till date. Out of the above CneAfr1p [41,42] and CneMdr1p [43] are the only two which have been found linked to the antifungal drug resistance in C. neoformans. CneAfr1p shows a high homology to A. nidulans AtrBp, AfuAtrFp, ScSnq2p, and CgPdh1p. Studies conducted on the cross resistance between amphotericin B and fluconazole in C. neoformans and C.gattii portray the defects in Erg2p (CneErg2p) or CneErg3p and additionally had reduced levels of ergosterol thus explaining the AMB resistance [44].
MFS Transporters
MFS transporters, encompasses a large superfamily of protein sharing a high sequence similarity all across the living world. The two subfamilies of MFS transporters have been classified on the basis of the number of transmembrane spans (TMS) within the transmembrane domain (TMD). The drug:H+ antiporter DHA1 has 12 TMS and DHA2 has 14 TMS [45]. In case of Candida albicans, out of the six genes (MDR1, FLU1, TPO3, orf19.2350, NAG3, and MDR97) annotated as MFS in the Candida genome database (CGD) only CaMdr1p and CaFlu1p have antifungal substrates. CaMdr1p is a type of DHA1 MFS transporter, and has been shown to confer resistance against fluconazole and ketoconazole, but not to miconazole or itraconazole [46].CaMdr1p over expression in C. albicans also imparts resistance to cerulenin and brefeldin A. Further, studies on structural and functional analyses of CaMdr1p determined a critical region in the TMS5 which is responsible for the drug/H+ transport [47]. FLU1 is another example of DHA1 MFS gene from C. albicans [48]. Studies suggest that not much on fluconazole susceptibility was visible by deletion of the FLU1 in C. albicans but this sensitized the cells to mycophenolic acid suggesting its activity as a pump substrate. In a study based on mutagenesis, 21 itraconazole resistant mutants were developed in which about half of the mutants, depicted resistance due to over expression of the efflux pumps AfuMDR3, an MFS-type transporter belonging to the DHA2 family.
The above highlighted transporters and the genes overexpressed in course of providing resistance against drugs can prove to be potential targets for adjunct therapy, involving killing the fungi and stopping them to achieve resistance against the main drug.
Conclusion
Growing resistance of the fungi against currently used antifungals is a matter of big concern for the scientific community. The rate of developing resistance against the antifungals is higher than the rates at which the new antifungals are introduced. Therefore, it is the need of the hour to understand the mechanism of the antifungal resistance and try to evade it. To address the same, this article underlines the various mechanisms of the antifungal resistance acquired by the fungi with a special emphasis on the transport based alterations. ABC proteins and MFS pumps both differing in the mechanism by which they harvest energy. ABC transporters are basically dependent upon the ATP phosphorylation for energy whereas MFS pumps depend upon the proton motive force for the same. Further, the different ABC and MFS transporters present in different species such as Candida, Aspergillus, Cryptococcus etc have been described along with their role in the antifungal drug resistance. This article will pay a pivotal role in understanding the major genes involved in inducing the resistance in different fungi and will definitely be helpful in conducting future studies on the resistance gained by fungi.
Acknowledgement
The author would like to thank “Archives of Biotechnology and Biomedicine” for this invited article. Further, Indian Council of Medical Research is also acknowledged for providing senior Research Fellowship to Mr. Neelabh.
References
- Pianalto KM, Alspaugh JA. New Horizons in antifungal therapy.J Fungi.2016; 4: 26. Ref.: https://goo.gl/Ay5dYZ
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. Hidden killers: human fungal infections.Sci Transl Med. 2012; 165: 165. Ref.: https://goo.gl/33Aymv
- Chu DT, Plattner JJ, Katz L. New directions in antibacterial research.J Med Chem. 1996;39: 3853-3874. Ref.: https://goo.gl/VCmMK4
- Kontoyiannis DP, Lewis RE. Antifungal drug resistance of pathogenic fungi. Lancet. 2002; 359: 1135-1144. Ref.: https://goo.gl/439pW7b
- Sanglard D, Coste A, Ferrari S. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation.FEMS Yeast Res. 2009;9: 1029-1050. Ref.: https://goo.gl/zEHzqX
- Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, et al. Efflux-mediated antifungal drug resistance.Clin Microbiol Rev. 2009; 22: 291-321. Ref.: https://goo.gl/FFooGK
- Bauer BE, Wolfger H, Kuchler K. Inventory and function of yeast ABC proteins: about sex, stress, pleiotropic drug and heavy metal resistance.Biochim Biophys Acta. 1999; 1461: 217-236. Ref.: https://goo.gl/go9UVs
- Decottignies A, Goffeau A. Complete inventory of the yeast ABC proteins.Nat Genet. 1997; 15: 137-145. Ref.: https://goo.gl/xcUV5X
- Sipos G, Kuchler K. Fungal ATP-binding cassette (ABC) transporters in drug resistance & detoxification.Curr Drug Targets. 2006; 7: 471-481. Ref.: https://goo.gl/4A6ijR
- Balzi E, Wang M, Leterme S, Van Dyck L, Goffeau A. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1.J Biol Chem. 1994; 269: 2206-2214. Ref.: https://goo.gl/U3bTgk
- Bissinger PH, Kuchler K. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product. A yeast ABC transporter conferring mycotoxin resistance. J Biol Chem. 1994; 269: 4180-4186. Ref.: https://goo.gl/iz8FPk
- Hirata D, Yano K, Miyahara K, Miyakawa T. Saccharomyces cerevisiae YDR1, which encodes a member of the ATP-binding cassette (ABC) superfamily, is required for multidrug resistance.Curr Genet. 1994;26: 285-294. Ref.: https://goo.gl/wHgpxt
- Sanglard D, Kuchler K, Ischer F, Pagani JL, Monod M, et al. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters.Antimicrob Agents Chemother. 1995; 39: 2378-2386. Ref.: https://goo.gl/NEkJb6
- Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors.Antimicrob Agents Chemother. 1996; 40: 2300-2305. Ref.: https://goo.gl/mNijQx
- Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene.Microbiology. 1997: 143: 405-416. Ref.: https://goo.gl/LWV1ux
- White TC. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997; 41: 1482-1487. Ref.: https://goo.gl/qJpXFb
- White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans.Antimicrob Agents Chemother. 2002; 46: 1704-1713. Ref.: https://goo.gl/4YHXtY
- Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms.J Antimicrob Chemother. 2002; 49: 973-980. Ref.: https://goo.gl/FBHFNd
- Balan I, Alarco AM, Raymond M. The Candida albicans CDR3 gene codes for an opaque-phase ABC transporter. Journal of bacteriology. 1997: 179. 7210-7218. Ref.: https://goo.gl/gfpPgY
- Franz R, Michel S, Morschhäuser J. A fourth gene from the Candida albicans CDR family of ABC transporters.Gene. 1998; 220: 91-98. Ref.: https://goo.gl/oDtXD3
- Cui Z, Hirata D, Miyakawa T. Functional analysis of the promoter of the yeast SNQ2 gene encoding a multidrug resistance transporter that confers the resistance to 4-nitroquinoline N-oxide. Biosci Biotechnol Biochem. 1999; 1: 162-167. Ref.: https://goo.gl/GMGyVG
- Oliveira K, Haase G, Kurtzman C, Jo J, Stender H. Differentiation of Candida albicans and Candida dubliniensis by fluorescent in situ hybridization with peptide nucleic acid probes. J Clin Microbiol. 2001; 11: 4138-4141. Ref.: https://goo.gl/wSd1Tk
- Borst A, Raimer MT, Warnock DW, Morrison CJ, Arthington-Skaggs BA. Rapid acquisition of stable azole resistance by Candida glabrata isolates obtained before the clinical introduction of fluconazole.Antimicrob Agents Chemother.2005; 2: 783-787. Ref.: https://goo.gl/J9CgcQ
- Sanguinetti M, Posteraro B, Fiori B, Ranno S, Torelli R, et al. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance.Antimicrob Agents Chemother.2005; 2: 668-679. Ref.: https://goo.gl/yHRQdL
- Miyazaki H, Miyazaki Y, Geber A, Parkinson T, Hitchcock C, et al. Fluconazole resistance associated with drug efflux and increased transcription of a drug transporter gene, PDH1, in Candida glabrata.Antimicrob Agents Chemother.1998; 7: 1695-1701. Ref.: https://goo.gl/cWM5wU
- Sanglard D, Ischer F, Calabrese D, Majcherczyk PA, Bille J. The ATP binding cassette transporter GeneCgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob Agents Chemother. 1999; 11: 2753-2765. Ref.: https://goo.gl/bti7d3
- Nakayama H, Izuta M, Nakayama N, Arisawa M, Aoki Y. Depletion of the squalene synthase (ERG9) gene does not impair growth of Candida glabrata in mice.Antimicrob Agents Chemother.2000; 9: 2411-2418. Ref.: https://goo.gl/wJERXi
- Fukuoka T, Johnston DA, Winslow CA, de Groot MJ, Burt C, et al. Genetic basis for differential activities of fluconazole and voriconazole against Candida krusei. Antimicrob Agents Chemother. 2003; 4: 1213-1219. Ref.: https://goo.gl/6DGpJh
- Orozco AS, Higginbotham LM, Hitchcock CA, Parkinson T, Falconer D, et al. Mechanism of Fluconazole Resistance in Candida krusei. Antimicrob Agents Chemother. 1998; 10: 2645-2649. Ref.: https://goo.gl/aNv2HZ
- Venkateswarlu K, Denning DW, Kelly SL. Inhibition and interaction of cytochrome P450 of Candida krusei with azole antifungal drugs.J Med Vet Mycol.1997; 1: 19-25. Ref.: https://goo.gl/n36Rwe
- Moran GP, Sanglard D, Donnelly SM, Shanley DB, Sullivan DJ, et al. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis.Antimicrob Agents Chemother.1998; 7: 1819-1830. Ref.: https://goo.gl/Mf42BU
- Barchiesi F, Calabrese D, Sanglard D, Di Francesco LF, Caselli F, et al. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750.Antimicrob Agents Chemother.2000; 6: 1578-1584. Ref.: https://goo.gl/dpRzv1
- Katiyar SK, Edlind TD. Identification and expression of multidrug resistancerelated ABC transporter genes in Candida krusei. Med Mycol. 2001; 1: 109-116. Ref.: https://goo.gl/fsrs5z
- Slaven JW, Anderson MJ, Sanglard D, Dixon GK, Bille J, et al. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate.Fungal Genet Biol.2002; 3: 199-206. Ref.: https://goo.gl/Lt1m6A
- Tekaia F, Latgé JP. Aspergillus fumigatus: saprophyte or pathogen?. Current opinion in Microbiology.2005; 4: 385-392. Ref.: https://goo.gl/rrDhR1
- Hu W, Sillaots S, Lemieux S, Davison J, Kauffman S, et al. Essential gene identification and drug target prioritization in Aspergillus fumigatus.PLoS Pathog.2007; 3: 24. Ref.: https://goo.gl/wk5Asm
- Andrade AC, Del Sorbo G, Van Nistelrooy JG, De Waard MA. The ABC transporter AtrB from Aspergillus nidulans mediates resistance to all major classes of fungicides and some natural toxic compounds. Microbiology.2000; 8: 1987-1997. Ref.: https://goo.gl/9KKDbR
- Andrade AC, Van Nistelrooy JGM, Peery RB, Skatrud PL, De Waard MA. The role of ABC transporters from Aspergillus nidulans in protection against cytotoxic agents and in antibiotic production.Mol Gen Genet.2000; 6: 966-977. Ref.: https://goo.gl/SiYTHx
- Angermayr K, Parson W, Stöffler G, Haas H. Expression of atrC-encoding a novel member of the ATP binding cassette transporter family in Aspergillus nidulans-is sensitive to cycloheximide.Biochim Biophys Acta. 1999; 2: 304-310. Ref.: https://goo.gl/omCCgD
- Del Sorbo G, Andrade AC, Van Nistelrooy JGM, Van Kan JAL, Balzi E, et al. Multidrug resistance in Aspergillus nidulans involves novel ATP-binding cassette transporters. Mol Gen Genet. 1997; 4: 417-426. Ref.: https://goo.gl/L8y1jg
- Posteraro B, Sanguinetti M, Sanglard D, La Sorda M, Boccia S, et al. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter‐encoding gene, CnAFR1, involved in the resistance to fluconazole.Mol Microbiol.2003; 2: 357-371. Ref.: https://goo.gl/XtZ598
- Sanguinetti M, Posteraro B, La Sorda M, Torelli R, Fiori B, et al. Role of AFR1, an ABC transporter-encoding gene, in the in vivo response to fluconazole and virulence of Cryptococcus neoformans.Infect Immun.2006; 2: 1352-1359. Ref.: https://goo.gl/VJYGGv
- Thornewell SJ, Peery RB, Skatrud PL. Cloning and characterization of CneMDR1: a Cryptococcus neoformans gene encoding a protein related to multidrug resistance proteins.Gene.1997; 1: 21-29. Ref.: https://goo.gl/qsedCC
- Venkateswarlu K, Taylor M, Manning NJ, Rinaldi MG, Kelly SL. Fluconazole tolerance in clinical isolates of Cryptococcus neoformans.Antimicrob Agents Chemother.1997; 4: 748-751. Ref.: https://goo.gl/PnUwMR
- Gbelska Y, Krijger JJ, Breunig KD. Evolution of gene families: the multidrug resistance transporter genes in five related yeast species.FEMS Yeast Res.2006; 3: 345-355. Ref.: https://goo.gl/sbwmT6
- Lamping E, Monk BC, Niimi K, Holmes AR, Tsao S, et al. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryot Cell. 2007; 7: 1150-1165. Ref.: https://goo.gl/Ts2gMZ
- Pasrija R, Banerjee D, Prasad R. Structure and function analysis of CaMdr1p, a major facilitator superfamily antifungal efflux transporter protein of Candida albicans: identification of amino acid residues critical for drug/H+ transport.Eukaryot Cell. 2007; 3: 443-453. Ref.: https://goo.gl/TfvHHj
- Calabrese D, Bille J, Sanglard D. A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole.Microbiology.2000; 11: 2743-2754. Ref.: https://goo.gl/7rQfQY