More Information
Submitted: May 18, 2021 | Approved: July 01, 2021 | Published: July 02, 2021
How to cite this article: Luisetto M, Edbey K, Mashori GR, Yesvi AR, Latyschev OY. OPEN and CLOSED state of SPIKE SARS-COV-2: relationship with some integrin binding. A biological molecular approach to better understand the coagulant effect. Arch Biotechnol Biomed. 2021; 5: 049-056.
DOI: 10.29328/journal.abb.1001028
Copyright License: © 2021 Luisetto M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: SPIKE; RGD; Integrin; Coagulation; Quantitative effect; Molecular approach
OPEN and CLOSED state of SPIKE SARS-COV-2: relationship with some integrin binding. A biological molecular approach to better understand the coagulant effect
Luisetto M1*, Khaled Edbey2, Mashori GR3, Yesvi AR4 and Latyschev OY5
1IMA Marijnskaya Academy, Applied Pharmacologist, Natural Science Branch, 29121 Italy
2Professor, Department of Chemistry, University of Benghazi, Libya
3Professor, Department of Medical & Health Sciences for Woman, Peoples University of Medical and Health Sciences for Women, Pakistan
4Founder and President, Yugen Research Organization, Undergraduate Student, Western Michigan University, MI, 49008 USA
5IMA Academy President, RU
*Address for Correspondence: Luisetto Mauro, IMA Marijnskaya Academy, Applied Pharmacologist, Natural Science Branch, 29121, Italy, Tel: +393402479620; Email: [email protected]
Related the physio-pathological process of COVID-19 disease it is interesting to focus to the aspect.
Played by interaction of Sars-Cov-2 protein with integrins of human epithelial pulmonary cell.
A bio molecular approach help in to deeply verify the involved factors and the results of this Activation RGD mediated.
Of Great interest also the relationship with some vaccine strategy followed by the various pharmaceutical industry.
The results of this work will be useful to think modification in some vaccine increasing the global safety and related some rare ADR.
Observing literature related SARS-COV 2 penetration in pulmonary epithelial cells it is reported a binding between Spike protein and ACE 2 receptor (primary way) but other mechanism seem involved like by some integrin binding (Trough the RGD spike domain).
Sourav Biswas, Vikram Thakur, Parneet Kaur, Azhar Khan, Saurabh Kulshrestha, Pradeep Kumara, Blood clots in COVID-19 patients: Simplifying the curious mystery. According article published in Med Hypotheses. 2021 doi: 10.1016/j.mehy.2020.110371
It was written:
Surprisingly, the autopsies of COVID-19 patients have revealed clots in the small -vessels of the lungs, heart, liver, and kidney which are responsible for strokes and heart -attacks. More than 33% of critical COVID-19 patients’ are reported with critically high- levels of blood -clotting or elevated levels of D-dimer.
We can hypothesize and end with the conclusion that the mysterious -clots reported in the COVID-19 patients may be due to the binding of the spike- protein of SARS-CoV-2 with the ACE2 receptor expressed in the endothelial-cells of blood vessels which may cause, vaso-constriction and activation of the intrinsic- pathway of coagulation and eventually results in the formation of blood-clots. in COVID-19 patients, the SARS-CoV-2 mediated endothelial- inflammation, thrombin generation, platelet, and leukocyte recruitment, complement activation, and the initiation of innate and adaptive immune- responses, forming clots, culminate in immune-thrombosis, ultimately resulting in thrombotic- complications, stroke, and finally death“.
And According article: practice/health-related behavior, or be reported in news media as established information.
Edward R. Kastenhuber, Javier A. Jaimes, Jared L. Johnson, Marisa Mercadante, Frauke Muecksch, Yiska Weisblum, Yaron Bram, Robert E. Schwartz, Gary R. Whittaker, Lewis C. Cantley. Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry. Preprint. doi: https://doi.org/10.1101/2021.03.31.437960
Evolutionary perspective on viral-host interaction
“Proteolytic -cleavage of the spike forms a barrier to zoonotic- crossover independent of receptor binding. Hemostasis is of central importance in mammals and represents a major vulnerability of mammals to predators and pathogens, either through hyper-activation of coagulation or uncontrolled-bleeding. The dysregulation of hemostasis is a convergent mechanism of toxins of snakes, bees, and bats and a driver of virulence in Ebola and dengue virus infection disease. Perhaps, SARS-CoV-2 has undergone selection to both induce and exploit an environment locally enriched in coagulation -proteases, instigating a positive- feedback loop to promote entry into additional host-cells”.
In an Interesting way Makowski, et al. written: “Numerous signaling -pathways are mediated by integrins and virion binding could lead to dys-regulation of these pathways, with consequent tissue- damage. Integrins on the surfaces of pneumocytes, endothelial cells and platelets may be vulnerable to CoV-2 virion- binding. Binding of virions to integrins on endothelial-cells could activate angiogenic- cell signaling pathways; dysregulate integrin-mediated signaling pathways controlling developmental-processes; and precipitate endothelial-activation to initiate blood- clotting. Such a procoagulant state, perhaps together with enhancement of platelet-aggregation through virions binding to integrins on platelets, could amplify the production of micro-thrombi that pose the threat of pulmonary thrombosis and embolism, strokes and other thrombotic consequences. With viral- invasion, the activated endothelium would cause platelet adhesion and activation with coincident switching of integrin αIIbβ3 into a high-affinity conformation. Fibrinogen binds to activated-integrin αIIbβ3 and is quickly converted to fibrin by the proteolytic activity of thrombin on the platelet. Surface. Platelet aggregation is then driven by binding of monomeric, oligomeric and polymeric fibrins to integrin αIIbβ3 molecules on adjacent platelets, linking them to one another in a rapidly growing-clot”.
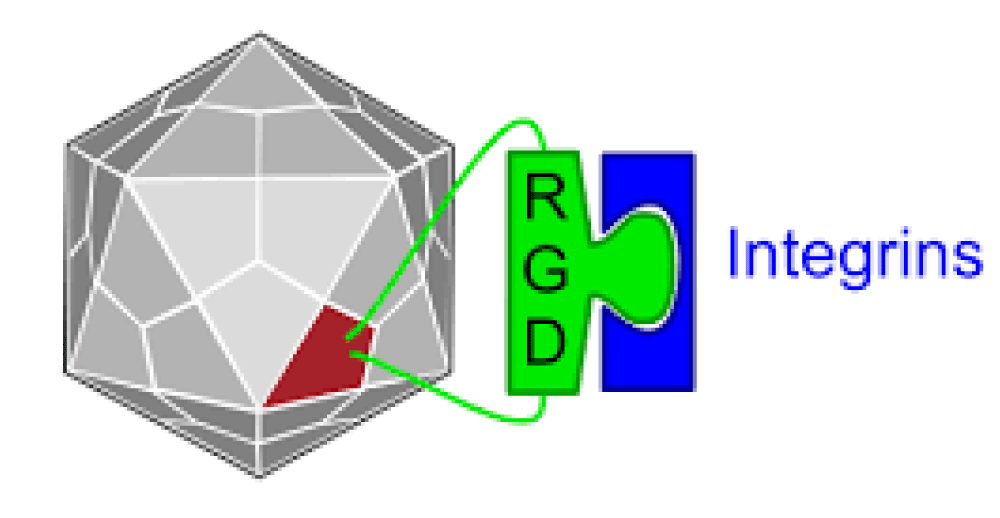
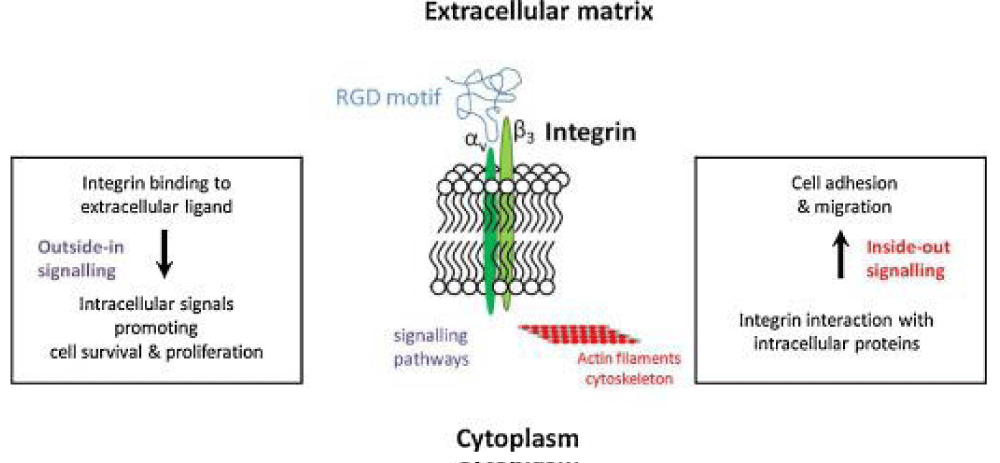
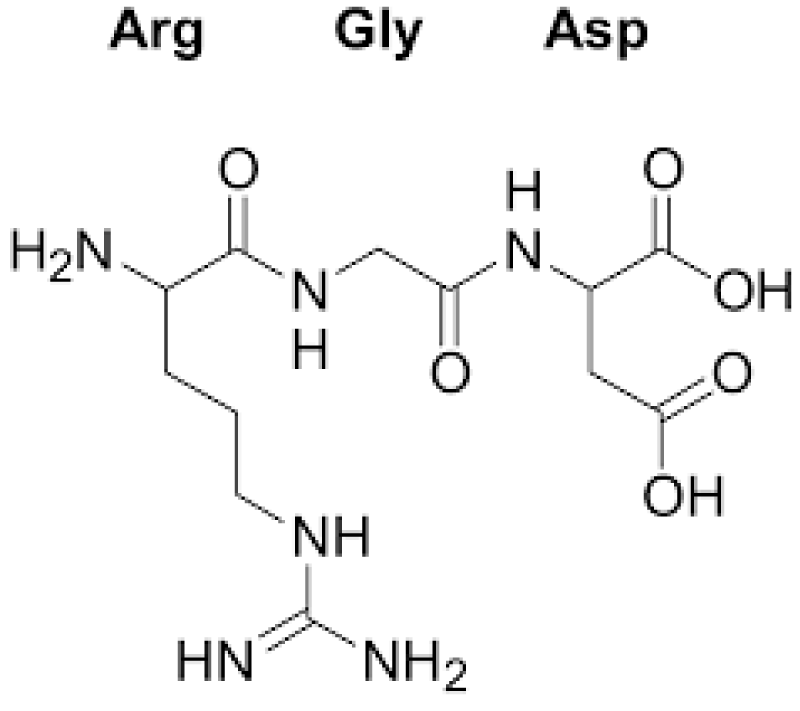
Registry of standard biological parts: Integrins are trans-membrane- proteins that, among other functions, mediate cell -attachment to surrounding tissues. They bind to a motif consisting of the amino acids arginine, glycine and aspartic acid (RGD). Because Integrin is highly expressed in many tumor- cell lines, AAV particles displaying the RGD motif on various positions in their capsid -proteins have been created by. Particles displaying RGD at amino acid positions 584 & 588 as well as 453 or 587 showed transduction- efficiencies similar to wt AAV, even when the cells’ HSPG -receptors were blocked by heparin -sulfate or when the natural HSPG binding motif on the capsid surface was knocked out.
Cold Spring Harb Perspect Biol. 2012 Feb; 4(2): a005132. doi: 10.1101/cshperspect.a005132
Extracellular Matrix Proteins in Hemostasis and Thrombosis
Wolfgang Bergmeier and Richard O. Hynes “platelets also express other ECM- receptor integrins at levels comparable to their levels of α2β1. These other integrins are α5β1, a receptor for fibronectin and fibrillin, and αvβ3, a promiscuous- receptor that can bind almost any ECM. Protein containing an exposed RGD motif ( thrombospondin, vitronectin, nidogens, fibronectin, fibrillin, and the major platelet- ligands, VWF and fibrinogen).
Based on this literature reported it is interesting to deeply investigate in relationship between the interaction Spike Sars cov-19 and some integrin, the efficacy of this binding and its activation effect.
The quantitative aspect it is relevant and crucial in order to translate to vaccine strategic design.
With an observational method some relevant and recent biomedical literature useful for the scope of this work is reported and analyzed.
Some figures (1-9) are reported to better explain the meaning of this work.
Al literature comes from Pub med or other opens literature journals.
Great enphasys is given to molecular biology aspects.
An experimental project hypotesys is submitted to the researcher in order to produce a global conclusion.
Figure 1: From Biological and Clinical Consequences of Integrin Binding via a Rogue RGD Motif in the SARS CoV-2 Spike Protein by Lee Makowski, William Olson-Sidford, John W. Weisel.
Figure 2: From http://parts.igem.org/Part:BBa_K404203
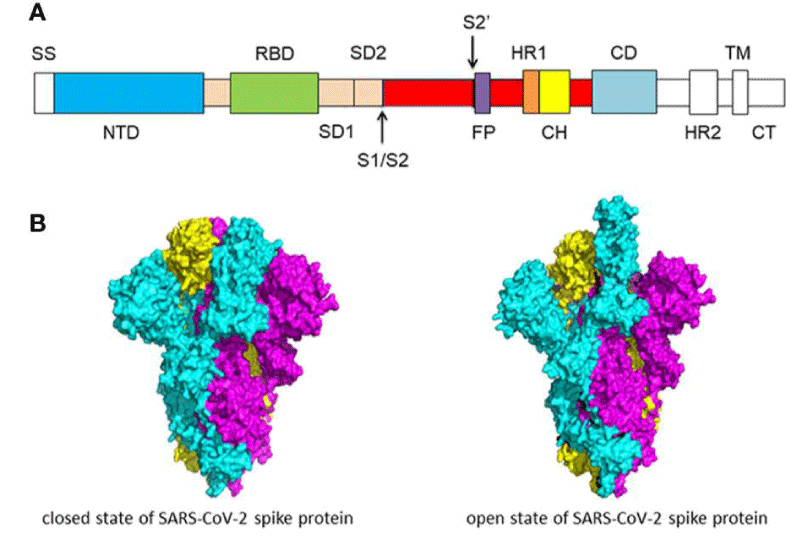
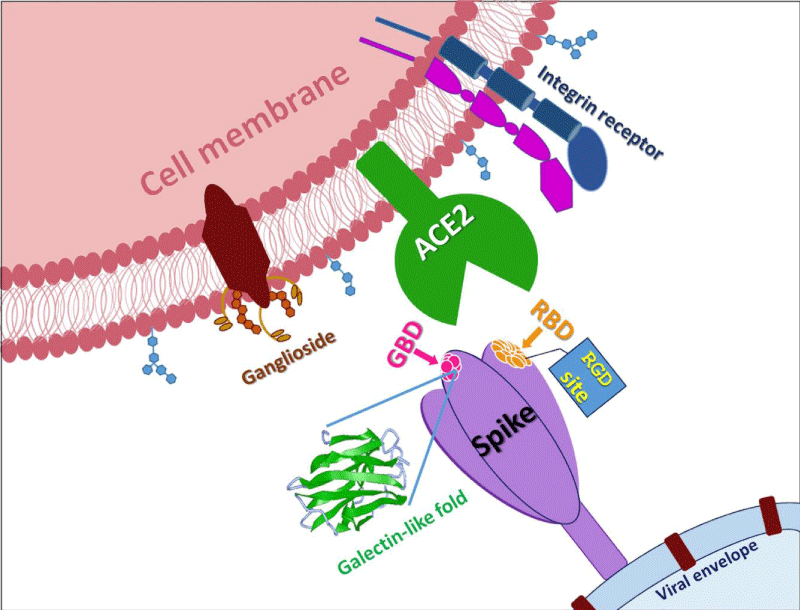
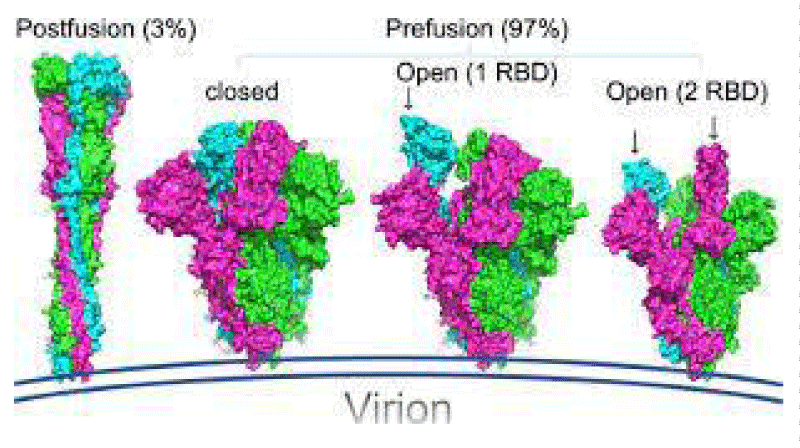
Figure 3: (A) Schematic of SARS-CoV-2 spike- protein primary- structure. Different domains are shown by different colors. SS, single- sequence; NTD, N-terminal domain; RBD, receptor-binding domain; SD1, subdomain 1; SD2, sub-domain 2; S1/S2, S1/S2 protease cleavage- site; S2’, S2’ protease cleavage site; FP, fusion peptide; HR1, heptad repeat 1; CH, central helix; CD, connector -domain; HR2, heptad repeat 2; TM, transmembrane- domain; CT, cytoplasmic tail. The protease cleavage site is indicated by arrows. (B) Cryo-EM structure of the SARS-CoV-2 spike protein. The closed- state (PDB: 6VXX) of the SARS-CoV-2 S glycoprotein (left) the open state (PDB: 6VYB) of the SARS-CoV-2 S glycoprotein (right).
Figure 3b: Gadanec LK, Mc Sweeney KR, Qaradakhi T, Ali B, Zulli A, et al. Can SARS-CoV-2 Virus Use Multiple Receptors to Enter Host Cells? Institute for Health and Sport, Victoria University, Melbourne 3030, Australia. Int J Mol Sci. 2021; 22: 992; https://doi.org/10.3390/ijms22030992
Figure 4: Luciano Pirone, Annarita Del Gatto, Sonia Di Gaetano, Michele Saviano, Domenica Capa, Laura Zaccaro and Emilia Pedone. A Multi-Targeting Approach to Fight SARS-CoV-2 Attachment. Front. Mol. Biosci., 03 August 2020 | https://doi.org/10.3389/fmolb.2020.00186.< 0.05).
Figure 5: Hustinx R. Integrin αvβ3 and RGD-based radiopharmaceuticalsL’intégrine αvβ3 et l’imagerie par les radiopharmaceutiques de type RGD. Médecine Nucléaire. 2016; 40: 41-54.
Figure 6:Pfaff M. Recognition Sites of RGD-Dependent Integrins. In: Integrin-Ligand Interaction. Springer, Boston, MA. 1997; https://doi.org/10.1007/978-1-4757-4064-6_4.
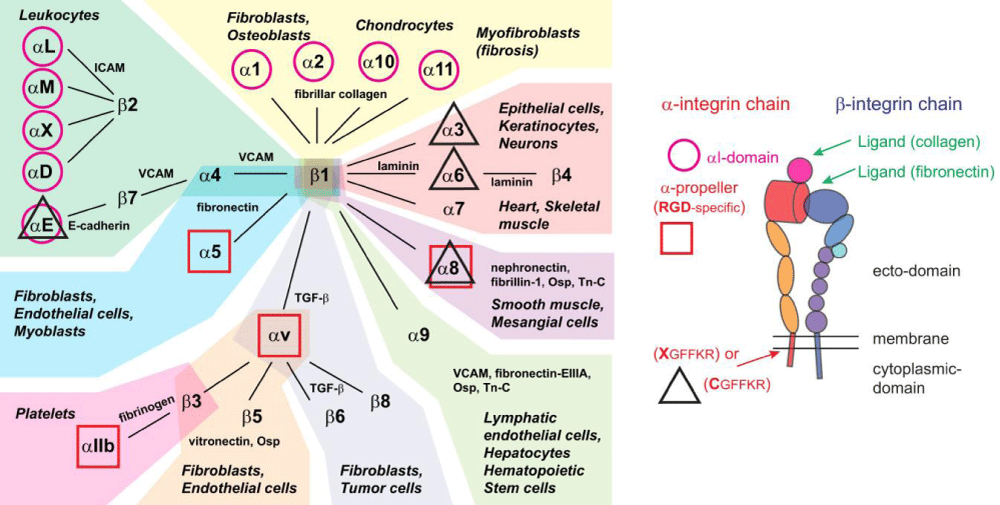
Figure 6b:Integrins, their ligands, and cellular distribution. According Hynes: integrins are organized in 24 different hetero-dimers, indicated by the link between α- and β-subunits in the figure. Integrins can be classified by structural -features, their ligands, and their tissue and cellular- expression. Based on these criteria, we grouped integrins into 9 classes indicated by the color of their background. Typical cell types expressing the respective integrins are mentioned, like common ligands for these integrins. We also highlighted integrins with an α-I domain (I; purple circle), those binding to RGD- ligands (RGD; red square), and those with changes to the conserved GFFKR sequence in the membrane-proximal part of the α-subunit (black triangle indicates integrins with sequence deviating from CGFFKR). The integrin- cartoon in the bottom part of the figure gives an overview about the integrin -structure and is reused in the following figures. It also indicates the location of the αI domain and the GFFKR sequence in the respective integrins. Please note that α-I domain integrins bind ligands (collagen) via this I domain, while other integrins bind ligands (fibronectin) in binding -pockets formed by both α- and β-subunits. Qiao B, de la Cruz MO. Enhanced Binding of SARS-CoV-2 Spike Protein to Receptor by Distal Polybasic Cleavage Sites. ACS Nano. 2020; 14: 10616–10623. https://doi.org/10.1021/acsnano.0c04798
Figure 7: Ismail AM, Elfiky AA. Luan J. SARS-CoV-2 spike behavior in situ: a Cryo-EM images for a better understanding of the COVID-19 pandemic. Signal Transduction and Targeted Therapy. 2020; 5: 252. Doi: https://doi.org/10.1016/j.jinf.2020.03.046.
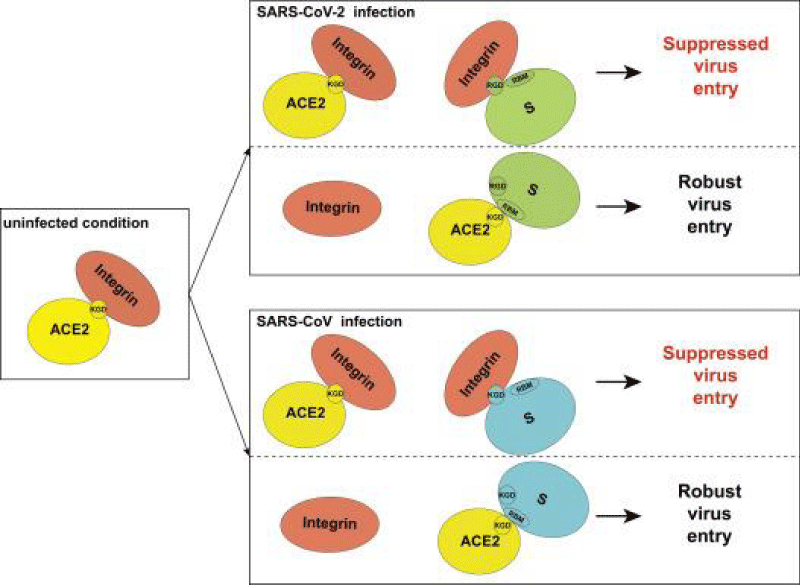
Figure 8: Proposed model for the inhibitory role of integrin in SARS-CoV-2 and SARS-CoV.
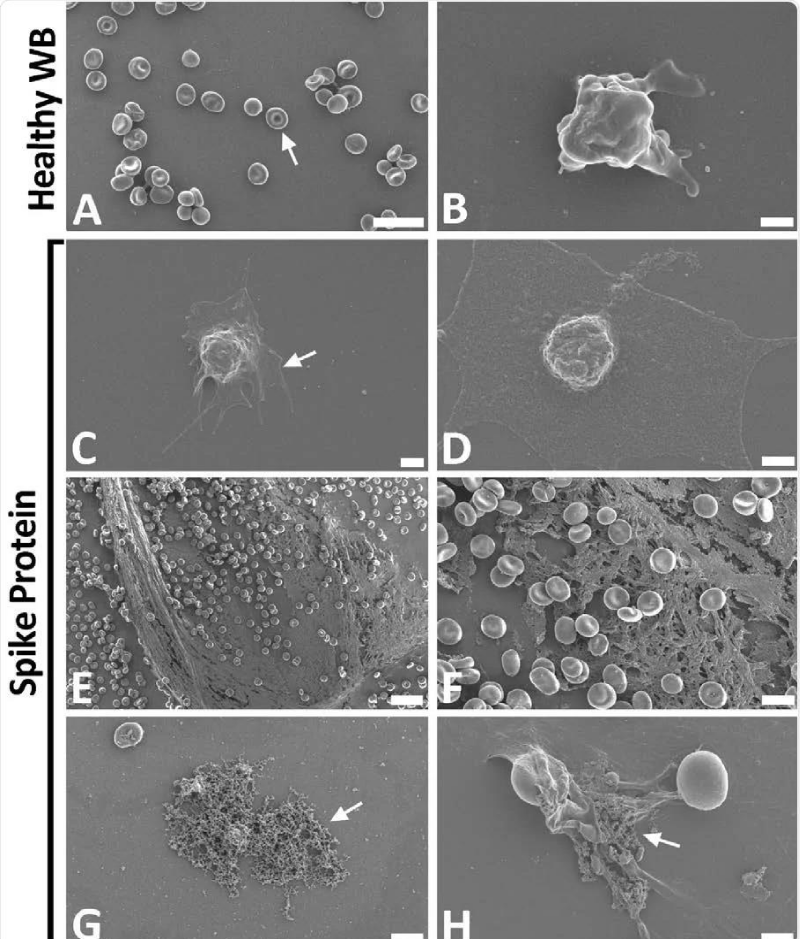
Figure 9: Scanning electron- micrographs of healthy control whole- blood (WB), with and without spike protein. A and B) Healthy WB smears, with arrows indicating normal erythrocyte- ultrastructure. C to H) Healthy WB exposed to spike- protein (1 ng.mL-1 final concentration), with C and D) indicating the activated -platelets (arrow), E and F) showing the spontaneously formed fibrin- network and G and H) the anomalous deposits that is amyloid in nature (arrows) (Scale bars: E: 20μm; A: 10μm; F and G: 5μm; H: 2μm; C: 1μm; B and D: 500nm). Form article SARS-CoV-2 spike S1 subunit induces hyper-coagulability SARS-CoV-2 spike protein S1 induces fibrin (ogen) resistant to the fibrinolysis: Implications for microclot formation in COVID-19 Lize M. Grobbelaar, Chantelle Venter, Mare Vlok, Malebogo Ngoepe, Gert Jacobus Laubscher, Petrus Johannes Lourens, Janami Steenkamp Douglas B. Kell, Etheresia Pretorius Preprint. Wool GD, Miller JL. The Impact of COVID-19 Disease on Platelets and Coagulation Pathobiology 2021; 88: 15–27.
From literature
Coagulopathy in COVID-19: Focus on vascular thrombotic events.
Wei Shi, Jiagao Lv, Li Lin, Published: July 15, 2020.DOI: https://doi.org/10.1016/j.yjmcc.2020.07.003
“Coagulation characteristics of COVID-19. Based on data extracted from COVID-19 patients during the first 2 months of viral outbreak, 59.6% severe cases showed a feature of elevated D-dimer (threshold as 0.5 mg/L) and average platelet-count in severe-COVID-19 patients appeared to be lower than that in non-severe group, which attracted much attention on coagulo-pathy secondary to COVID-19. A retrospective cohort-study enrolling 191 cases was published at March 11th, which introduced the clinical course and risk -factors for adult deaths in COVID-19 patients in Wuhan, China. In this study, coagulation -dysfunction was found in 27 (50%) non-survivors of COVID-19, with elevated D-dimer and prolonged PT. In another work, with 183 cases enrolled and dynamic alterations in coagulation profiles recorded, Tang et al. reported a mortality of 11.5% in COVID-19 patients and they found 15 (71.4%) of the deaths ascribed to COVID-19 met the diagnostic -criteria of overt disseminated intravascular- coagulation (overt-DIC, ≥5 points) based on the international society on thrombosis and haemostasis (ISTH), but, only 0.6% of the survivors matched the criteria” [1].
“S-protein is composed of two functional -subunits, including the S1 and S2 subunits. The S1 subunit consists of N-terminal domain (NTD) and receptor binding- domain (RBD). The function of S1 subunit is bind to the receptor on host cell. S2 subunit contains fusion- peptide (FP), heptad repeat 1 (HR1), central helix (CH), connector domain (CD), heptad repeat 2 (HR2), trans-membrane- domain (TM), and cytoplasmic tail (CT). The function of S2 subunit is to fuse the membranes of viruses and host- cells. The cleavage site at the border between the S1 and S2 subunits is called S1/S2 protease -cleavage site. For all the coronaviruses, host proteases cleave the spike- glycoprotein at the S2’ cleavage site to activate the proteins which is critical to fuse the membranes of viruses and host -cells through irreversible conformational changes. N-linked glycans are critical for proper folding, neutralizing antibodies, and decorating the spike- protein trimers extensively.
The S -protein has two forms of structure, including the closed state and the open- state. In the closed state, the three recognition motifs do not protrude from the interface formed by three spike- protein protomers. In the open state, the RBD is in the “up” conformation. The open state is necessary for the fusion of the SARS-CoV-2 and the host cell membranes, thereby facilitating SARS-CoV-2 to enter the host- cells.
One study work obtained a 3.5 Å-resolution structure of spike- protein trimer with one RBD in the in the “up” conformation (receptor-accessible state). Receptor- binding destabilizes the prefusion structure, triggered by this process, the S1 subunit dissociates and the S2 subunit refolds into a stable post-fusion conformation, which has been captured in SARS-CoV. RBD goes through conformational -transitions like a hinge, leading to the hide or exposure of the determinants of the spike- protein to engage a host -cell receptor. This process will form the following 2 states: “down” conformation and “up” conformation. In the “down” conformation, SARS-CoV-2 could not recognize the ACE2 on the host- cells. The structure of SARS-CoV-2 is highly similar with SARS-CoV. One of the larger differences is in the down -conformation, SARS-CoV RBD packs tightly against the NTD of the neighboring protomer, while the angle of SARS-CoV-2 RBD is near to the central- cavity of the spike -protein trimer. When aligned the individual structural- domains corresponding to SARS-CoV-2 and SARS-CoV, highly similar structures were observed” [1] (Figure 3).
“HCoVs are positive-sense, single-stranded RNA- viruses and 30,000 bp long. 2 types of proteins are identified in HCoVs: four structural- proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins and non-structural proteins, like proteases (nsp3 and nsp5) and RNA dependent-RNA polymerase (nsp12). The S- protein is a pivotal -tool for virus- adhesion and entry into host -cells and it can represent an intriguing target for the development of antibodies, entry inhibitors or vaccines. It is present on the virion’s outer surface and displays a homo-trimeric state.
Protein S allows viral entrance into host- cells by firstly binding to a host -receptor, such interaction occurring through the receptor binding -domain (RBD) in subunit S1, and secondly through subunit S2 the viral and host -membranes fuse. Similar to SARS-CoV, SARS-CoV-2 also recognizes angiotensin-converting enzyme 2 (ACE2) as its host receptor-binding.The variation of crucial residues in S- protein of SARS-CoV-2 may contribute to the highly- transmission efficiency of the virus. The reported evolutionary- mutation of K403R in S1 protein of SARS-CoV-2 forms an RGD motif in RBD at the interaction -surface neither been found in Bat RaTG13 nor in SARS-CoV. The RGD motif is the cell attachment site of a great amount of adhesive extracellular- matrix and cell surface proteins and is recognized by the membrane receptor- integrins. The integrins are hetero-dimers of α- and β-subunits linked in a non-covalent manner and play key functions in cell -adhesion, cell-cell interactions, signaling and defense- mechanisms. Human meta-pneumo-virus is similar to SARS-CoV-2 regarding the organ tropism and symptoms, and its protein F’s RGD triade displays a fold comparable to that of SARS-CoV-2. Integrins play an important role in different respiratory -diseases, in particular in pneumonia resulting from bacteria or virus- infections. Upon the binding, the RBD of S1 subunit undergoes a conformational shift and this change exposes or hides the key- region of binding -domain to access to ACE2. The RGD motif would be exposed to the surface of the host cell- membrane with the key binding region and thus prones to interact with integrin. Biomedical-Literature data show that also ACE2 displays a conserved RGD motif and it is able to bind integrin- subunits. The binding seems to be RGD-independent because of the in-accessibility of the motif, letting to speculate that thanks to a conformational- shift the site could be exposed, enhancing cell adhesion and therefore justifying the higher infectivity” [2].
Eleazar Ramírez Hernández, Luis Fernando Hernández-Zimbrón, Nayeli Martínez Zúñiga, Juan José Leal-García, Violeta Ignacio Hernández, Luis Eduardo Ucharima-Corona, Eduardo Pérez Campos, and Edgar Zenteno. The Role of the SARS-CoV-2 S-Protein Glycosylation in the Interaction of SARS-CoV-2/ACE2 and Immunological Responses. According: Viral Immunology. Vol. 34, No. 3 16 Apr 2021. https://doi.org/10.1089/vim.2020.0174
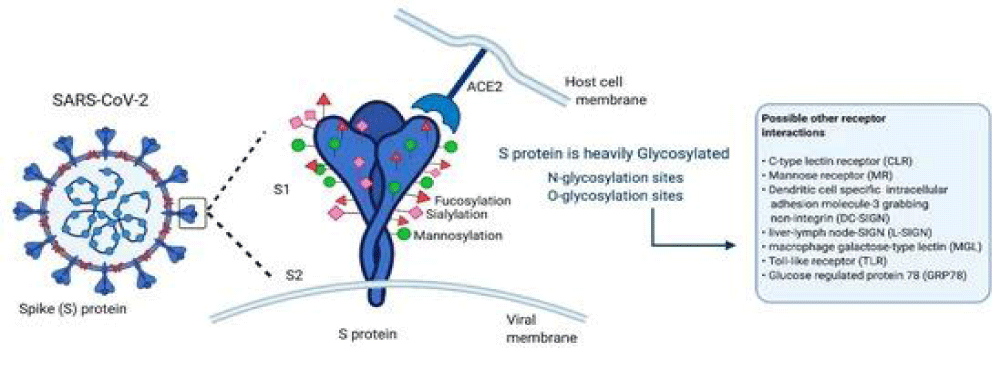
“The glycans displayed on the viral- surface are added during replication inside the host. On the contrary, glycosylation of the virus is critical for maintaining the stability of these proteins and the viral-particles, as suggested for flavi-viruses, including dengue or Zika, or for S glycoproteins from influenza- A virus, coronavirus (SARS-CoV), Ebola-virus, among others The S glycoprotein of SARS-CoV-2 plays a relevant role in infecting the host-cells and in its transmission- capacity. The S- glycoprotein is a type I transmembrane protein of 1255 amino acids AA, which is a trimer in the prefusion and post-fusion conformations; the cryo-EM analysis has confirmed this structure for both conformations. This glycoprotein comprises 2 subunits responsible for host cell receptor binding (S1 subunit) and fusion of the viral and cellular membranes (S2 subunit). The SARS-CoV-2 S glycoprotein contains 22 N-linked glycosylation sequons per protomer; the map of oligo-saccharides has been resolved by cryo-EM for 16 of the sites and, experimentally, it has been confirmed that ∼19 of them are glycosylated. 20 of the 22 N-linked glycosylation- sequons of SARS-CoV-2 S are conserved in SARS-CoV-1 S. 9 of 13 glycans in the S1 -subunit and all 9 glycans in the S2 subunit are conserved between SARS-CoV-2 S and SARS-CoV-1 S.
S2 subunit N-linked glycosylation is mainly conserved in SARS-CoV S glycol-proteins, indicating that the availability of the viral- fusion machinery is comparable between these viruses. Recent evidence has been published showing low- levels of O-glycosylation in SARS-S protein. These oligo-saccharides contribute to S protein- folding, impinge on priming by host proteases, and regulate antibody recognition.
Low levels of O-linked glycosylation have been detected, suggesting that O-glycans of this region are insignificant when the structure is native- like. The presence of O-glycans in some viral proteins suggests an important role in biological-activity. In the SARS-CoV-2 S1, the O-type glycosylation by O-GalNAc and O-GlcNAc seems to be involved in protein stability and function. The S glycoprotein is a target in vaccine design; the changes in the glycosylation of viral-spikes can disclose important elements for the knowledge of viral-biology and facilitate vaccine- design strategies. Glycoproteins with a high density of glycans can facilitate the camouflaging of immunogenic epitopes and promote immune-evasion.
General review
“The tripeptide motif Arg-Gly-Asp (RGD) was identified more than 12 years ago as a minimal essential peptide- sequence inhibiting cell -adhesion to fibronectin.1–3 Subsequently, short RGD-peptides were found to inhibit the binding of other major cell-adhesive proteins like vitronectin, fibrinogen or van- Willebrand factor, indicating the central biological- importance of this simple peptide motif as the basic unit of a widespread cellular recognition -system.”
According: Michael Bachmann, et al. Cell Adhesion By Integrins. Physiol Rev. 2019; 99: 1655–1699. https://journals.physiology.org/doi/pdf/10.1152/physrev.00036.2018
“Only a few years later it became clear that fibronectin was a major extracellular binding-partner for fibroblasts and that the critical binding -element in fibronectin was a short peptide Arg-Gly-Asp (RGD).
The I-domain insert of the α-subdomain of the lymphocyte -integrins (αM) was crystallized in 2 different conformations, providing a strong argument for the association of integrin ligand- binding with conformational changes in the receptor. While the I-domain of the α-subunit exhibited a single metal-ion-dependent ligand binding -site, the revelation of the structure of the entire extracellular- domain of the αvβ3 integrin receptor, identifying three differently complexed metal- ions coordinating the RGD- peptide to the central Mg2+ ion, determined a breakthrough in understanding how integrin-ligand-binding was coupled to conformational changes of the integrin -receptors. The structural differences between the headpiece of the lymphocyte-integrin αM and the integrin αvβ3 expressed in fibroblasts and endothelial cells allowed first considerations about the connection of integrin structure to physiological -function.
Given the frequency of the RGD- sequence in many extracellular matrix- proteins, the group of RGD-binding integrins is considered to recognize many different ligands.
“ACE2 is highly negatively charged (−28 e), which leads to a highly negatively charged extra-cellular membrane surface. The Coulomb- interactions favor the adsorption of positively- charged species, for instance, arginine residues. Due to the positively -charged nature, arginine has been found to enhance the cellular- uptake of peptides”
We identified an RGD/KGD integrin-binding motif in S- proteins from SARS-CoV-2 and SARS-CoV. We also discovered a KGD integrin-binding motif in ACE2. Integrins were predicted to inhibit receptor targeting of S proteins from SARS-CoV-2 and SARS-CoV by shielding both S- protein and ACE2.
Integrins are hetero-dimeric proteins comprising α and β subunits in cell- surface. Many integrins recognize Arg-Gly-Asp (RGD) and Lys-Gly-Asp (KGD) motifs which are displayed on the exposed- loops of proteins. RGD could associate broader types of integrins such as αVβ1, αVβ3, αVβ5, αVβ6, αVβ8, αIIbβ3, αMβ2, αLβ2 and α3β1, while KGD-recognizing integrins are restricted to αIIbβ3, αVβ5, αVβ6 and αVβ8.2” [3].
According J. CALVER: “Crucially αvβ3 integrins are up-regulated in COVID-19 infected lung- tissue, whereas ACE2 levels remain low even in patients with high viral- RNA and protein- expression in alveolar -tissue.
Our data suggests SARS-CoV-2 is able to bind integrins, and may utlise this mechanism to facilitate internalization into lung epithelial- cells, which may help explain severe pathology despite low ACE2 expression- levels in the lung” [4].
“We demonstrated that COVID-19 patients present with increased mean platelet- volume (MPV) and platelet hyper-activity, which correlated with a decrease in overall platelet- count. Detectable SARS-CoV-2 RNA in the blood stream was associated with platelet hyper-activity in critically ill patients” [5].
“As reported by numerous research work, grave SARS-CoV-2 infection is frequently complicated with coagulopathy, and thrombo-embolic events are recognizable in several patients. In a retrospective study, 260 out of 560 subjects (46.4%) with laboratory proved SARS-CoV-2 disease had an increase of D-dimer, and the augment was more prominent among grave- patients (59.6% vs. 43.2%). The Authors argue that D-dimer alteration can indicate the gravity of the infection and an augmented concentration is correlated with a poorer- prognosis” [6].
“The viral load of 2019-nCoV detected from patient respiratory- tracts was positively linked to lung- disease severity” [7].
Zhang, et al. have very recently taken such analysis still further, reporting their detection of ACE2 expression by platelets, the direct stimulatory -effects of SARS-CoV-2 or its Spike- protein on platelets, and the ability of recombinant human ACE2 protein or anti-Spike monoclonal -antibody to inhibit Spike protein-induced platelet- activation.
“COVID-19 is associated with a pro-thrombotic phenotype characterised by coagulo-pathy and endothelial dysfunction. Following some cases of thrombosis after the vaccination, Oxford-Astra Zeneca+ vaccine (AZD1222) was temporarily suspended by some European -countries. The European medicines agency (EMA) concluded that the benefits of the vaccine in combating COVID-19 outbreak continue to outweigh the risk of side effects. On March 19, 2021, Germany reported 13 cases of sinus or cerebral vein -thrombosis with more than 1.6 million AstraZeneca COVID-19 vaccine doses administered. Some of these patients also had a heparin-induced thrombocytopenia (HIT)-like syndrome, which does suggest an immunological- event as one of the potential origin of thrombosis.we observed unexpected cerebral venous- thrombosis (CVT) for COVID-19 vaccine- Moderna (0.9% (3/325) of events reported; time to event: 2 to 39 days; range: 30–37 years-old; 3 women), for AZD1222 (1.1% (7/639) of events reported; time to event: 2 to 16 days; range: 19–59 years-old; 3 women and 4 men) and with the Comirnaty (0.4% (4/1197) of events reported; time to event: 1–10 days; range: 30–84 years-old; 4 women). Three patients out of four with Comirnaty, all Moderna vaccine and six out of seven with AZ1222 had a particular form of CVT, called cerebral sinus vein- thrombosis (CVST). Five out of seven CVT cases observed after AZD1222 were associated to thrombo-cytopenia” [8].
“In India, Bahrat Biotech’s vaccine, Covaxin (BBV152), was well tolerated by the participants in Phase I/II trials. Covaxin was given in a 2-dose regimen over 3 to 4 weeks and was able to induce robust humoral immune- responses in all the participants.
Four days after the first vaccination, the rapid humoral- responses were documented [9].
Katherine J. Wu. Dec. 31, 2020
Updated Feb. 8, 2021 The new York time Vaccines Take a While to Kick In. Experts Say That Means the Body Is Doing Its Job.
Reports of COVID-19 cases that appeared shortly after a single shot of a 2-dose vaccine shouldn’t cause concern.
Vaccines take at least a few days to exert their protective effects. Pfizer’s recipe is designed around a molecule called messenger RNA, or mRNA, which, once injected, enters human -cells and instructs them to manufacture a coronavirus- protein named spike. None of these components are infectious or capable of causing Covid-19. But they act as coronavirus mimics, teaching the body to recognize the true -virus and vanquish it, should it ever come around.
The production of spike is thought to occur within hours of the first shot. But the body needs at least several days to memorize the material before it can unspool its full-arsenal of defensive -forces against the virus. Immune-cells take this time to study up on the protein, then mature, multiply and sharpen their spike-spotting reflexes.
Experimental project hypotesys.
In order to verify in vitro the quantitative relationship between sars-covid-19 spike protein concentration.
It is interesting to verify at various concentration (low, medium, high level) the effect on a plasma and observing also the platelet activation (clotting activity).
The concentration must to be chosen observing the pulmonary tissue level of spike protein in various class of patient (severe or not severe) and related viral load measured.
The same it must to be verified related the amount of spike trasduced by the various kind of vaccine.
All result must to be numeric data to produce a graph model and to obtain a curve of relationship (if present). In this case the test must to be done at various time t zero, t1, t2, t3 after vaccination (observe in literature the days of the rare thrombosys happened in some vaccinated patient).
T1= 1-2 days after vaccination
T2= 5-7 days after vaccination
T3 = after 10-30 days
And so on but also after 6 month.
Useful to do this is to observe epidemiology of the rare thrombosis related the most frequent days of start after the vaccine under investigation.
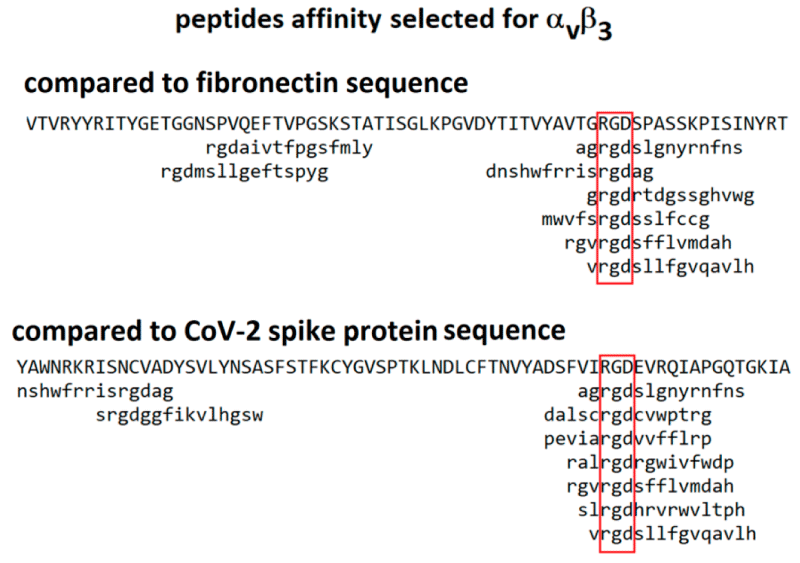
In this article are reported figure and reference also related RGD motif of spike protein and the ability of this to link also some integrin like fibronectin.
The same literature link viral load to a pro-trombotic status in the moderate severe covid-cases.
Integrin like fibronectin is physiologically involved in coagulation process as reported by many scientific literature (since many years showed a proved).
But what is relevant is to verify if there is a quantitative relationship between spike protein and procoagulant status and to do this verify of relationship between the viral load (quantitative measured) and the Trombosys event is crucial (The same also to verify the platelet level).
The same it is interesting to verify the level of spike trasduced after a vaccination (for all class of vaccine) and to compare this with the COVID severe disease level.
Researcher verify that the covid-19 virus not only bind ACE2 receptor but also some integrins like fibronectin.
In this process The RGD motif play a crucial role and also the status of OPEN or CLOSED to bind in efficient way (more or less).
But what is relevant is to observe the real clinical implication: there is a specific level for activation dose dependent?
Scientist find relationship between covid-19 viral load (laboratory tested quantitative methods) and severity For disease and related the activation of blood coagulation.
Finally it is interesting to observe that in some RARE adverse event of some covid-19 vaccine a trombosys event was observed (see report of EU pharmacovigilance).
So in order to proof or not this properties it is opinion of the authors that it is needed to verify in vitro the procoagulant effect level after COVID-19 Vaccination ( for all the various class of vaccine) at time t zero, T1, T2, T3 to verify if a quantitative Relationship whit the transcripted SPIKE level il present or not.
Novelty: this article introduce a quantitative aspect in spike related procoagulant activity RGD mediated.
This aspect is relevant also for COVID -vaccine design of new-or next generation.
Ethical consideration: all international rules respects.
- Mei-Yue W, Zhao R, Li-Juan G, Xue-Fei G, De-Ping W, et al. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front Cell Infect Microbiol. 2020; 10: 587269. PubMed: https://pubmed.ncbi.nlm.nih.gov/33324574/
- Pirone L, Del Gatto A, Di Gaetano S, Saviano M, Capasso D, et al. A Multi-Targeting Approach to Fight SARS-CoV-2 Attachment. Front Mol Biosci. 2020; 7: 186. PubMed: https://pubmed.ncbi.nlm.nih.gov/32850973/
- Luan J, Lu Y, Gao S, Zhang L. A potential inhibitory role for integrin in the receptor targeting of SARS-CoV-2. 2020; 81: 318-356. PubMed: https://pubmed.ncbi.nlm.nih.gov/32283163/
- Calver J, Joseph C, John AE, Organ L, Fainberg H, et al. Understanding lung infection: back to basics. S31 The novel coronavirus SARS-CoV-2 binds RGD integrins and upregulates avb3 integrins in Covid-19 infected lungs. Thorax BMJ. 2021; 76: A22-A23.
- Zhang S, Liu Y, Wang X, Yang L, Li H, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020; 13: 120. PubMed: https://pubmed.ncbi.nlm.nih.gov/32887634/
- Allegra A, Innao V, Allegra AG, Musolino C. Coagulopathy and thromboembolic events in patients with SARS-CoV-2 infection: pathogenesis and management strategies. Ann Hematol. 2020; 99: 1953-1965. PubMed: https://pubmed.ncbi.nlm.nih.gov/32671455/
- Liu Y, Yang Y, Zhang C, Huang F, Wang F, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020; 63: 364-374. PubMed: https://pubmed.ncbi.nlm.nih.gov/32048163/
- Smadja DM, Qun-Ying Y, Chocron R, Sanchez O, Louet AL. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J. 2021; 2100956. PubMed: https://pubmed.ncbi.nlm.nih.gov/33863748/
- Loo KY, Letchumanan V, Ser HL, Teoh SL, Law JWF, et al. COVID-19: Insights into Potential Vaccines. Microorganisms. 2021; 9: 605. PubMed: https://pubmed.ncbi.nlm.nih.gov/33804162/
- Grobbelaar LM, Venter C, Vlok M, Ngoepe M, Laubsche GJ, et al. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19. 2021.