More Information
Submitted: January 20, 2025 | Approved: January 30, 2025 | Published: January 31, 2025
How to cite this article: Azadeh SS, Neghab HK. The Role of Mitochondria in Chronic Wound Healing (Mitotherapy): Signaling and Therapeutic Implications. Arch Biotechnol Biomed. 2021; 9(1): 001-009. Available from:
https://dx.doi.org/10.29328/journal.abb.1001043.
DOI: 10.29328/journal.abb.1001043
Copyright License: © 2025 Azadeh SS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Mitotherapy; Mitochondria; Wound healing; Hypoxia
The Role of Mitochondria in Chronic Wound Healing (Mitotherapy): Signaling and Therapeutic Implications
Seyedeh Sara Azadeh* and Hoda Keshmiri Neghab
Department of Medical Laser, Medical Laser Research Center, Yara Institute, ACECR, Tehran, Iran
*Address for Correspondence: Seyedeh Sara Azadeh, Department of Medical Laser, Medical Laser Research Center, Yara Institute, ACECR, Tehran, Iran, Email: [email protected]
Mitochondria are essential intracellular organelles that significantly influence various cellular processes, including metabolism, stress response, and cell fate. Their precise regulation is crucial for maintaining both organelle and cellular homeostasis. Wound healing is a complex, multifactorial process that relies on the coordinated actions of multiple cell types and numerous cellular mechanisms. Dysregulation in this process can lead to chronic wounds, which pose substantial challenges for healthcare systems and present limited treatment options due to their intricate pathogenesis. Recent research has increasingly focused on the role of mitochondria in wound healing, revealing their involvement in critical processes such as metabolism, apoptosis, and redox signaling. Mitochondrial dynamics play a vital role in wound healing by adapting to cellular demands and environmental cues. Moreover, mitophagy, the selective degradation of damaged mitochondria, is crucial for maintaining mitochondrial integrity and function during the healing process. Mitochondria are not only pivotal in energy production but also in calcium homeostasis and the generation of mitochondrial reactive oxygen species, which are essential for signaling during wound repair. As wound healing progresses through distinct yet overlapping stages mitochondria facilitate the energy demands of repair and contribute to cytoskeletal remodeling necessary for wound closure. Understanding the multifaceted roles of mitochondria in wound healing could lead to novel therapeutic approaches for chronic wounds. Future research should prioritize investigating mitochondrial dynamics and functions in human tissues to develop targeted strategies for enhancing wound healing outcomes.
Wound healing is a fundamental biological process that ensures the restoration of tissue integrity and homeostasis following injury. This intricate and highly regulated mechanism consists of four overlapping phases: hemostasis, immune response/inflammation, proliferation, and tissue remodeling. Each phase plays a critical role, and any disruption (whether in isolation or collectively) can lead to dysfunctional healing and the formation of chronic wounds [1]. Defined as wounds that do not heal within three months or fail to progress through the expected phases despite treatment, chronic wounds pose significant challenges to healthcare systems, accounting for approximately 2%–4% of global healthcare budgets [2]. Understanding the pathogenesis of chronic wounds is essential for developing effective treatment strategies. The wound healing process shares similarities with tissue repair mechanisms in other organs, such as muscle, liver, and bone. This suggests that insights gained from studying wound healing could have broader implications for understanding healing processes in various disease contexts. Mitochondria, the energy-producing organelles within eukaryotic cells, play a pivotal role in the metabolic demands of wound healing [3]. Originating from an ancient α-proteobacterium through endocytosis nearly two billion years ago, mitochondria have evolved into essential components of cellular function. Human mitochondria contain their double-stranded genome (mtDNA), which encodes critical proteins involved in the Electron Transport Chain (ETC) and various metabolic pathways. In addition to mtDNA, thousands of nuclear-encoded polypeptides contribute to the mitochondrial proteome, highlighting the complexity of mitochondrial function in cellular processes [4]. Given the metabolic intensity of wound healing and the critical involvement of mitochondria, a deeper exploration of their role may provide valuable insights into chronic wound pathophysiology. This understanding could ultimately enhance therapeutic approaches, not only for wound healing but also for broader applications in regenerative medicine and tissue repair across various biological systems [5]. Mitochondria are often recognized primarily as the powerhouses of the cell, generating and storing energy in the form of Adenosine Triphosphate (ATP). However, their roles extend far beyond energy production. Mitochondria are integral to a variety of critical cellular processes, including stress response, immune function, redox balance, calcium homeostasis, and the regulation of cell fate [6]. Central to these functions is the concept of mito-nuclear crosstalk, a communication pathway whereby mitochondria sense stress signals and relay them to the nucleus, prompting a coordinated response that can influence mitochondrial structure and function. This feedback mechanism is crucial for maintaining mitochondrial homeostasis, encompassing changes in mitochondrial shape (dynamics) as well as adjustments in mitochondrial number through biogenesis and mitophagy [5].
The effectiveness of wound healing depends on the proper functioning of mitochondria, and mitochondrial dysfunction can lead to delayed or impaired healing, especially in chronic conditions such as diabetic wounds and radiation-induced wounds. This study explores the therapeutic potential of mitochondria and mitochondria-based treatments in improving wound healing. The table presented here summarizes key research on the role of mitochondria in wound healing, the various strategies employed in research, and the observed effects on wound healing. These strategies include methods such as mitochondrial transplantation, modulation of mitochondrial stress, and the use of innovative delivery systems to enhance mitochondrial function. The findings from these studies demonstrate the significant potential of mitochondria-focused therapies in improving wound healing outcomes, particularly in challenging conditions. This table aims to provide a comprehensive understanding of recent studies on the various mechanisms through which mitochondria influence wound healing and the therapeutic approaches that can be explored for future clinical applications (Table 1).
| Table 1: The Role of Mitochondria in Wound Healing and Mitochondria-Based Therapeutic Strategies. | |||
| Role of Mitochondria in Wound Healing | Research Actions | Observed Effects | Reference |
| Modulating the immune environment and controlling inflammation in wounds | Investigating the effect of membrane vesicles (MVs) secreted by Lactobacillus reuteri (RMVs) on wound healing |
Significant improvement in mucosal and cutaneous wound healing by modulating the inflammatory environment | [7] |
| Providing energy for various healing processes | Investigating the effect of transplanting healthy mitochondria from skeletal muscles to chronic pressure wounds | Reduction in wound size, increased granulation tissue, and accelerated epithelialization | [8] |
| Impact of mitochondrial stress on inflammation and immune regulation |
Studying the mechanism of ER-mitochondria interaction in the presence of LZD | Increased ER-mitochondria interaction, leading to activation of the inflammatory NLRP3 complex | [9] |
| Regulating inner mitochondrial membrane dynamics | Using sodium ionophores to increase intracellular Na+ content |
Increased keratinocyte proliferation and migration, enhanced angiogenesis | [10] |
| Regulating mitochondrial energy metabolism | Studying the effect of increased Na+ on mitochondrial proton (H+) metabolism in the matrix | Increased H+ in the mitochondrial matrix improved energy metabolism and accelerated wound healing | [9,11] |
| Identifying new pharmaceutical targets for diabetic wounds | Proposing Na+ ionophores as a new drug option | Monensin A identified as a potential agent for chronic skin wound treatment in diabetic patients | [12] |
| Counteracting mitochondrial autophagy dysfunction and iron-induced cell death in oxidative stress environments | Engineering pH and glucose-responsive Hst1@CBTC hydrogels with dynamic borate bonding | Peptide release of up to 90 ± 4.2% to improve mitochondrial function and cellular viability | [13] |
| Accelerating the healing of chronic diabetic wounds | Testing the effect of the hydrogel on diabetic wound models | Wound closure rate of 94.7 ± 5.1% in 14 days |
[12,14] |
| Exploring molecular pathways related to mitochondrial function improvement | Activation of the Nrf/HO1 pathway to reduce ferroptosis effects | Reduced oxidative stress and improved mitochondrial function | [15] |
| Developing safe biological materials for chronic wound treatment | Evaluating the safety and biocompatibility of the hydrogel | Introduction of a safe hydrogel made from natural materials for diabetic wound treatment | [16] |
In the context of wound healing, the multifaceted roles of mitochondria become particularly significant. The healing process relies on a well-orchestrated interplay of cellular mechanisms, and mitochondrial health is paramount for the normal functioning of these processes. Recently research underscores the involvement of mitochondria in various skin pathologies associated with impaired wound healing. This review aims to elucidate the specific contributions of mitochondria in regulating metabolism during wound healing. By focusing on these aspects, we will highlight the critical importance of mitochondria in maintaining cellular homeostasis and facilitating effective wound repair. Mitochondria are essential organelles in cells that carry out various critical functions, including energy production, calcium regulation, redox signaling, and metabolism. These functions are closely associated with the highly dynamic morphology of mitochondria, which can undergo rapid and transient changes. Such Adaptations enable cells to respond effectively to environmental stimuli and metabolic demands. I
In particular, mitochondrial morphology and activity are vital in physiological processes like wounds. N healing. Wound healing in mammals is a multifaceted process that necessitates the coordinated efforts of different cell types, progressing through distinct but overlapping phases: hemostasis and inflammation, cell proliferation and migration, and tissue remodeling. This cellular-level process lays the groundwork for tissue repair and regeneration. Recent studies have shown that mitochondria meet the high energy requirements for wound repair and facilitate wound closure through cytoskeletal remodeling, driven by morphological changes and signaling from mtROS. In this review, we will focus on how wounding triggers alterations in mitochondrial morphology and activity, and how these changes contribute to the cellular response and repair mechanisms associated with wounds. Additionally, this review will highlight recent findings and address existing gaps in our understanding of the role of mitochondria in wound healing and related processes, underscoring their significance as crucial components in these biological mechanisms.
Phases of wound healing
Wound healing is a complex and dynamic process that consists of four sequential and overlapping phases: hemostasis, inflammation, proliferation, and tissue remodeling. This intricate series of events is essential for restoring tissue integrity and ensuring homeostasis after an injury [17].
- Hemostasis: The initial phase, hemostasis, occurs immediately following wounding, where platelets are recruited to the site, leading to clot formation and vasoconstriction. This phase is crucial as it initiates the healing cascade and sets the stage for subsequent inflammatory responses [18].
- Inflammation: During the inflammation phase, which typically lasts from one to three days, the wound environment becomes hypoxic, triggering the activation of local immune cells. These immune responses involve the infiltration of leukocytes, including neutrophils and macrophages, which play vital roles in pathogen clearance and the release of proinflammatory mediators. This phase is characterized by a complex interplay of immune cells, signaling molecules, and growth factors, laying the groundwork for the healing process [19].
- Proliferation: The proliferation phase follows, spanning from four to twenty-one days post-injury, where significant cellular activities occur, including re-epithelialization, granulation tissue formation, and angiogenesis. Fibroblasts are the primary drivers of this phase, producing extracellular matrix components that form a scaffold for new tissue. Concurrently, keratinocytes proliferate and migrate to cover the wound, aided by growth factors that stimulate new blood vessel formation [19].
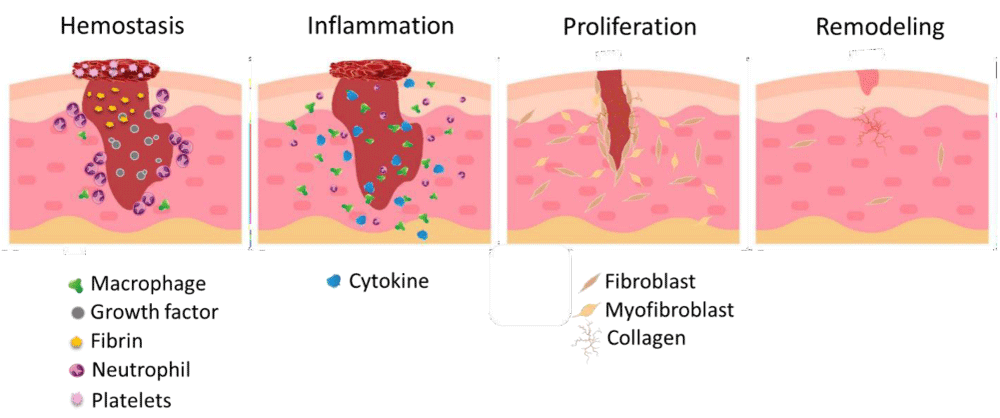
- Remodeling: The remodeling phase begins roughly three weeks after the injury and can last up to a year. During this phase, the wound undergoes contraction and maturation, as fibroblasts transform into myofibroblasts, facilitating the reorganization of collagen and the formation of scar tissue. This final stage aims to restore the normal structure and function of the tissue, although the resulting scar may differ in composition and appearance from the original skin (Figure 1) [20].
Figure 1: The wound healing process. These stages are hemostasis, which occurs within hours after the injury. Inflammation, lasting from 1 to 3 days post-injury. Proliferation, spanning from 4 to 21 days. Tissue repair can last from approximately 21 days up to a year. In the hemostasis phase, inflammatory cells, growth factors, and fibrin gather at the wound site, leading to clot formation and blood vessel constriction. During the inflammation phase, additional immune cells are activated, serving as immune responders and producing growth factors. In the proliferation phase, re-epithelialization takes place, along with the growth of new blood vessels and granulation tissue. Finally, in the remodeling phase, the wound undergoes contraction, resulting in the formation of scar tissue [21].
Overall, understanding the intricacies of these phases is critical for developing effective therapeutic strategies for improving wound healing outcomes, particularly in cases of chronic wounds that fail to progress through the normal healing process. Chronic wounds represent a significant healthcare challenge, characterized by their failure to heal despite appropriate medical intervention. These wounds can arise from various etiologies, including diabetes, neuropathies, genetic predispositions, and vascular insufficiencies, which contribute to their heterogeneous nature. The pathogenesis of chronic wounds is complex and multifactorial, with disturbances occurring at multiple stages of the wound-healing process. Notably, the inflammatory phase can be marked by either an exaggerated immune response or an inadequate ability to clear pathogens, leading to prolonged tissue inflammation that hinders healing [22]. Additionally, dysfunctions during the tissue repair phase, such as impaired angiogenesis and re-epithelialization, can create a hypoxic environment that exacerbates cell death and promotes necrosis [23]. Given the intricate interplay of factors contributing to chronic wound formation, a multidisciplinary approach is essential for effective treatment strategies. However, advancing knowledge in this field is challenging due to the limitations of existing preclinical models. While animal studies in species like pigs and mice offer insights into wound healing mechanisms, they do not fully replicate human pathophysiology, particularly regarding the processes of re-epithelialization and angiogenesis [23,24]. Invertebrate models, despite their utility in studying innate immune responses, lack the complexity necessary to investigate critical aspects of human wound healing. Recent technological advancements, such as single-cell transcriptomic, provide new avenues for exploring the underlying mechanisms of chronic wounds, particularly the role of mitochondria [25]. These organelles are emerging as crucial players in the wound-healing process, influencing cellular metabolism, inflammation, and tissue repair. To develop more targeted treatments and preventive measures for chronic wounds, further research into mitochondrial function and its implications in wound healing is essential. By enhancing our understanding of these cellular processes, we can pave the way for innovative therapeutic approaches to address chronic wound management effectively.
Mitochondria
Mitochondria are semi-autonomous organelles surrounded by double membranes in eukaryotic cells, primarily recognized for their function in producing ATP through Oxidative Phosphorylation (OXPHOS). The process of ATP synthesis relies on five protein complexes (Complexes I-V) located in the Inner Mitochondrial Membrane (IMM) and two mobile electron carriers. Besides their role in energy production, mitochondria are also significant sources of Reactive Oxygen Species (ROS), which are byproducts of OXPHOS mainly generated by Complexes I and III. These mtROS can enter the cytoplasm, facilitating redox signaling that influences various biological processes such as cell proliferation, differentiation, and migration [26,27]. Mitochondria exhibit remarkable plasticity, displaying a range of shapes from fragmented spheres to extensive reticular networks. This morphological variability is regulated by the opposing processes of fusion and fission. The mechanisms governing these processes are well-conserved, primarily involving Guanosine Triphosphatases (GTPases) from the dynamin family. Mitochondrial fission is primarily mediated by Dynamin-Related Protein 1 (DRP1) [28,29]. When fission is activated, this large cytosolic GTPase binds to the Outer Mitochondrial Membrane (OMM) through adaptor proteins like Mitochondrial Dynamics protein 49 (MiD49) and Fusion Protein 1 (FIS1), forming a ring structure that constricts and divides the parent mitochondrion into two daughter mitochondria. Additionally, research has shown that mitochondrial shape can change rapidly through other mechanisms, including actin-dependent fission and regulation by the Endoplasmic Reticulum (ER), FUNDC1, and myosin [30]. Mitochondrial Rho GTPase MIRO-1 has also been identified as a regulator of mitochondrial shape in various organisms, responding to cytosolic Ca²⁺ signals. Mitochondrial fusion, on the other hand, involves the merging of both the OMM and IMM, with mitofusins 1 and 2 (MFN 1 and MFN2) facilitating the outer membrane fusion and Optic Atrophy Protein 1 (OPA1) mediating inner membrane fusion. These morphological changes are closely tied to mitochondrial function, affecting ATP and mtROS production and enabling mitochondria to adapt to environmental changes and cellular requirements [28,31]. Wound healing is a crucial process for the survival of all multicellular organisms, particularly in mammals, where it involves a coordinated effort from various cell types and occurs through distinct but overlapping stages: hemostasis and inflammation, cell proliferation and migration, and tissue remodeling. The hemostatic phase begins immediately after injury, activating the coagulation cascade and sealing blood vessels to minimize blood loss. Concurrently, inflammatory cells such as neutrophils and macrophages are drawn to the wound to combat potential infections. Following this, epithelial cells proliferate, differentiate, and migrate to restore the damaged area. The process of single-cell wound repair resembles that of tissue repair, allowing insights into the cellular biology underlying tissue damage. Emerging evidence indicates that mitochondria are instrumental during these phases, with changes in their shape and activity playing significant roles. This review will primarily focus on how wounding triggers these mitochondrial morphological adaptations and how these alterations contribute to the cellular response and repair processes following injury [5,32].
Calcium ions affected Mitochondria morphology
Calcium ions (Ca²⁺) are crucial for the wound healing process, and mitochondria play a key role in regulating Ca²⁺ signaling within cells. Despite this, there is limited research specifically exploring the connection between mitochondria and Ca²⁺ in the context of wound healing. An increase in intracellular Ca²⁺ is one of the initial damage signals during wound healing and is vital for initiating and modulating the healing process [33]. As highlighted earlier in this review, the propagation of Ca²⁺ is involved in various functions, including serving as inflammatory mediators and influencing the proliferation, migration, and differentiation of fibroblasts and keratinocytes during re-epithelialization. Additionally, Ca²⁺ is crucial for angiogenesis in wound healing and can stimulate the production and release of mtROS. Ca²⁺ signaling pathways also play a role in regulating metabolism and the formation of the extracellular matrix [34].
As noted, mitochondria are primarily responsible for managing cellular Ca²⁺ storage and transport, and mitochondrial Ca²⁺ is essential for various signaling pathways and cellular energy production. Ca²⁺ enters mitochondria through the mitochondrial Ca²⁺ uniporter (MCU) and exits via the mitochondrial Na⁺/Ca²⁺/Li⁺ exchanger (NCLX). However, due to their low affinity for Ca²⁺, the transfer of Ca²⁺ into mitochondria occurs through close interactions with the endoplasmic reticulum, known as Mitochondrial-Associated Membranes (MAMs). These MAMs are important for numerous mitochondrial functions, including glucose sensing, mitophagy, apoptosis, the Unfolded Protein Response (UPR), insulin signaling, and ROS signaling [35]. Recent studies have highlighted the significant role of Ca²⁺ in managing oxidative stress, mitochondrial dynamics, and biogenesis, particularly in aiding reepithelialization during wound healing. Additionally, research by Parnis et al. demonstrated that maintaining mitochondrial calcium homeostasis is crucial for cell proliferation and wound healing in astrocytes [36]. However, much remains unknown about the role of mitochondrial Ca²⁺ homeostasis in skin wound healing, particularly regarding its influence on apoptosis and immune responses in humans. The increase in cytosolic Ca²⁺ around the wound margin is a conserved mechanism across different species. Ca²⁺ acts as one of the earliest transcription-independent signals in response to injury, activating downstream targets that facilitate various wound repair functions, including inflammation and actomyosin-regulated repair. The mechanisms behind Ca²⁺ elevation can vary; for instance, our lab studies the C. elegans syncytium epidermal cell hyp7 as a model for wound repair. In these cells, Ca²⁺ enters the extracellular space directly through Transient Receptor Potential (TRP) channels, such as GTL-2 [37,38]. In other cases, Ca²⁺ is released from internal stores like the endoplasmic reticulum via inositol-1,4,5-triphosphate (IP3) receptors activated by G-Protein-Coupled Receptor (GPCR) signaling. Regardless of the source, the rapid increase in intracellular Ca²⁺ and its wave-like propagation enable quick detection of wounds [38].
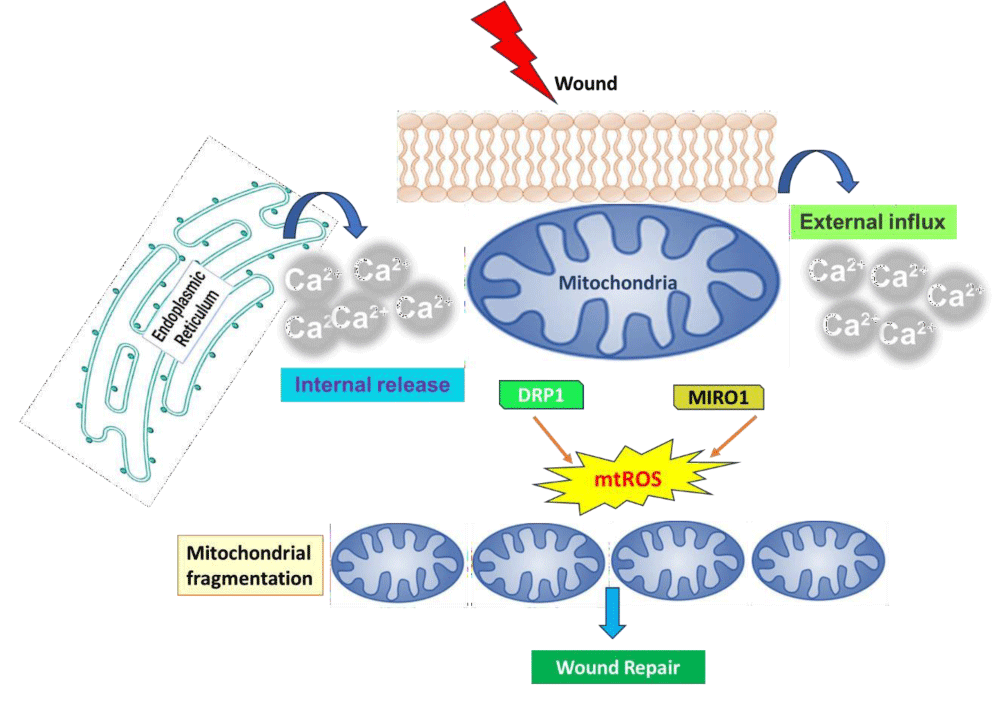
After injury, mitochondria undergo rapid and reversible fragmentation, which is dependent on the increase in cytosolic Ca²⁺. This response is spatially restricted, with fragmented mitochondria near the injury site being separate from the interconnected mitochondria farther away. The changes in mitochondrial shape following wounding depend on different molecular mechanisms across various cell types, likely due to inherent differences that affect the expression of specific regulators. The fragmentation of mitochondria after wounding is primarily driven by Dynamin-Related Protein 1 (DRP-1). If DRP-1 and its adaptor MiD49 are deleted in mammalian cells, the mitochondrial network becomes overly elongated and fails to fragment following localized membrane injury, leading to compromised membrane integrity. In Drosophila embryos with different drp-1 mutants, two distinct epidermal wound closure phenotypes are observed: one with a significantly slower closure rate and another with a larger wound area. However, the mechanisms by which DRP-1 detects damage and triggers mitochondrial division remain unclear [39]. Mitochondrial fragmentation is also seen in C.elegans after epidermal injury, where the mitochondrial network at the injury site first fragments, followed by fragmentation in surrounding mitochondria within minutes [40]. Interestingly, this fragmentation occurs independently of DRP-1, as both drp-1 knockdown and drp-1 deletion mutants show normal mitochondrial fragmentation after wounding. The study also finds that the spreading of mitochondrial fragmentation requires MIRO-1, an adaptor on the Outer Mitochondrial Membrane (OMM) crucial for mitochondrial transport. MIRO-1 acts as a Ca²⁺ sensor on the OMM and can directly detect the increase in Ca²⁺ resulting from wounding, which initiates rapid mitochondrial fragmentation [40]. MIRO-1 is involved in mitochondrial transport along microtubules and interacts directly with Trak-1, a kinesin protein that facilitates trafficking. However, knocking down track-1 in C. elegans does not affect the mitochondrial fragmentation induced by wounding. Additionally, inhibiting microtubule dynamics does not impact this fragmentation, indicating that the process is not dependent on microtubules [41]. The precise molecular mechanisms by which MIRO-1 regulates mitochondrial fragmentation still need clarification. It's possible that other mitochondrial fission machinery may interact with MIRO-1 to control fragmentation, or that MIRO-1 could directly influence this process. It is known that a loss of mitochondrial membrane potential leads to fragmentation; however, whether MIRO-1 is involved in regulating this potential is still unknown. In Drosophila embryos, DRP-1 facilitates injury-induced mitochondrial division, aligning with findings in mammalian cells. While both DRP1 and MIRO-1 are involved in regulating mitochondrial dynamics, it remains uncertain whether they function redundantly in response to injury or if there is any genetic or physical interaction between them. MIRO-1 is also vital for the regeneration of dendrites in PVD sensory neurons, which respond to high-threshold mechanical stimuli in C. elegans. Overall, these findings illustrate that Ca²⁺-dependent mitochondrial fragmentation is a common response to injury, facilitating efficient wound repair [36,41] (Figure 2).
Figure 2: A model illustrating the role of mitochondrial fragmentation and reactive oxygen species (ROS) signaling in wound repair highlights the various functions of mitochondria in both single-cell closure and multicellular healing. When tissue is wounded, there is a rapid increase in cytosolic calcium (Ca²⁺) due to external influx or internal release, which triggers mitochondrial fragmentation through the actions of DRP-1 or MIRO-1. In response to this calcium increase, fragmented mitochondria enhance the production of mtROS, aiding in the closure of the actin ring in single-cell systems. Additionally, mtROS may support multicellular wound healing by participating in processes such as angiogenesis and inflammation [42].
Mitochondria Dynamic in wound healing
Mitochondria can quickly adjust to meet changing metabolic needs within a cell or respond to stress. Generally, an increased demand leads to enhanced mitochondrial biogenesis (mitogenesis) and the fusion of mitochondria to expand their network. Conversely, decreased demand or specific stressors can trigger mitochondrial fission and, in some cases, lead to degradation through autophagy (mitophagy) [43]. Mitochondria are in a constant state of fission and fusion, a process known as “mitochondrial dynamics.” These dynamics influence mitochondrial morphology, including their number, size, distribution, transport, and quality control. Proper management of these factors is crucial for maintaining mitochondrial function. Over recent decades, research has demonstrated that mitochondrial dynamics play a significant role in various cellular processes such as proliferation, cell-cycle progression, calcium (Ca²⁺) homeostasis, and cell death. Dysregulation of these dynamics is linked to several diseases. The protein Drp1 serves as the primary regulator of mitochondrial fission, being recruited to the Outer Mitochondrial Membrane (OMM) by receptors like Fis1, Mff, MIEF1, and MIEF2. Upon recruitment, Drp1 wraps around the mitochondria and induces constriction via its GTPase activity. For mitochondrial fusion, three key proteins—Opa1, Mfn1, and Mfn2—are involved. Mfn1 and Mfn2, located on the OMM, form a dock between adjacent mitochondria before facilitating fusion through GTP hydrolysis. In addition to its fusion role, Mfn2 also participates in insulin signaling, energy metabolism, connections between the endoplasmic reticulum and mitochondria, and mitophagy. Opa1, located in IMM, is responsible for fusing the IMM and mitochondrial matrices of neighboring mitochondria [44]. Recent studies have indicated that mitochondrial fission, resulting in a fragmented mitochondrial phenotype characterized by more spherical, unfused mitochondria and a less tubular, branched network, is crucial for wound healing. In a study by Ponte et al., which examined wound healing in Drosophila with various mutations in mitochondrial dynamics genes, researchers found that Drp1-mediated fission and a fragmented mitochondrial phenotype regulated wound healing by controlling ROS production, Ca²⁺ homeostasis, and the accumulation of F-actin at the wound site. This study also showed that mutations in Fis1, which led to mitochondrial fragmentation, impaired wound healing. Similarly, research by Fu et al. demonstrated the importance of mitochondrial fission in wound healing in C. elegans and zebrafish but found that the fragmentation occurring immediately after wounding was independent of Drp1 and relied instead on the Rho GTPase.
MIRO-1 [40,41]. This fragmentation was associated with increased mtROS production, Ca²⁺ signaling, and more glycolytic metabolism. However, neither study investigated mitochondrial dynamics in human tissues, which may account for differences in findings. Additionally, while a few studies utilized mouse models, it is important to recognize the significant differences in wound healing between humans and mice, such as the presence of the panniculus carnosus and more frequent stem cell niches due to increased hair follicle density in mice. Nonetheless, these models provide valuable opportunities to study wound healing pathophysiology at a higher organismal level and highlight the necessity for studies on human tissues [45]. A lack of electron microscopy analysis in these studies also limits understanding of mitochondrial ultrastructure and cristae complexity in the context of wound healing. Previous research has shown that differentiating epidermal keratinocytes exhibit a fragmented mitochondrial phenotype. This fragmentation aligns with the decreased energy requirements of keratinocytes during differentiation, as opposed to proliferating basal keratinocytes, and facilitates the degradation of mitochondria necessary for keratinocyte differentiation [46]. Mitochondrial fragmentation is known to promote apoptosis in macrophages and support glucose homeostasis and glycolytic metabolism across various tissues. Although the role of mitochondrial fragmentation in these processes has not been specifically examined within the context of wound healing, it is well-established that proper regulation of these physiological processes is critical for effective wound healing.
Mitochondrial ROS facilitates wound healing
An increase in cytosolic calcium ions (Ca²⁺) triggers rapid uptake of mitochondrial calcium through the MCU, which aids in wound healing by temporarily boosting the production of mtROS. This response was first observed in the epidermis of C. elegans after injury using a mitochondrial superoxide sensor, mito::cpYFP, and was found to be absent in MCU-1 mutants. Although the exact mechanism for this increased mtROS production is not fully understood, it is believed that mitochondrial calcium uptake leads to the opening of the mitochondrial permeability transition pore and wounding-induced mitochondrial fragmentation [47]. Mitochondrial Ca²⁺ uptake enhances OXPHOS, which increases ATP production and its byproduct, mtROS. Interestingly, while ATP synthesis is not essential for skeletal muscle repair, mtROS has a dose-dependent beneficial effect on wound healing. These findings illustrate that the increase of mtROS in injury plays a critical role in cellular repair processes. However, the mechanism by which mitochondrial ROS signaling is localized to the injury site remains unclear [48]. ROS mainly originate from mitochondria, peroxisomes, and the endoplasmic reticulum, but can also be produced in the cytoplasm. Mitochondria and NADPH oxidases on the plasma membrane are the most well-characterized sources of intracellular ROS. Levels of ROS are necessary for cellular wound closure and various multicellular healing processes, including hemostasis, inflammation, and tissue remodeling. Researchers find that In C. elegans, epidermal wound healing requires the formation of an actin ring around the wound site through rapid actin polymerization, which is mediated by CDC42 and negatively regulated by RHO-1 [48]. The increase in mtROS at the wound site can promote repair; inhibiting mtROS with antioxidants prevents actin-based wound closure, while mutations that elevate mtROS enhance healing. mtROS can inhibit RHO-1 activity, which negatively influences actin ring closure. A similar mechanism is seen in injured skeletal muscle cells, where calcium triggers mtROS production, facilitating wound closure by activating RhoA to promote F-actin accumulation. Further research revealed that the upregulation of mtROS is necessary for faster wound closure in fzo-1 mutant C. elegans, which have constitutively fragmented mitochondria [40,48]. In fzo-1 mutants, increased mtROS production and the upregulation of several oxidative stress-related genes, including cytochrome P450 family genes, are observed after wounding. CYPs can produce ROS, which can, in turn, enhance CYP expression. RNA interference (RNAi) knockdown of cyp genes results in lower mtROS levels and inhibits actin ring closure, while their overexpression accelerates closure in both fzo-1 mutants and wild-type animals. Similarly, antioxidant treatments in fzo-1 mutants reduce ROS levels and delay wound closure, while treatment with the mitochondrial Complex I inhibitor rotenone, which induces ROS production, can reverse these effects [49]. During Drosophila dorsal closure, mtROS can initiate cell delamination. It has been found that mtROS can stimulate mitochondrial fragmentation through Drp1 and caspase activation and influence cytoskeletal rearrangement via the Rho effector ROCK and the pMLC pathway. In laser-wounded Drosophila embryonic epidermis, defects in mitochondrial fission lead to decreased mtROS levels and slower wound closure, suggesting that elevated mtROS levels are crucial for enhanced wound healing in the epidermis of C. elegans [40]. MtROS also plays a role in maintaining homeostasis in damaged tissue, mediating vasoconstriction and thrombus formation. Antioxidant treatment can inhibit vasoconstriction under cold exposure in mice, and ROS is involved in this process through the ROS/RhoA/ROCK1 and ROS/PKC/ET-1 pathways in vascular smooth muscle and endothelial cells. Respiratory deficiency restricted to the vasculature through cox10 ablation results in embryonic lethality in mice, while in adults, cox10 deficiency leads to impaired angiogenesis near wounds. A cell-permeable mitochondrial ubiquinol–cytochrome c reductase binding protein can enhance mtROS production, which induces vascular endothelial growth factor expression, promoting angiogenesis and cutaneous wound healing in mice [38]. MtROS is also responsible for producing pro-inflammatory cytokines following lipopolysaccharide stimulation in normal cells and excessive cytokine production in cells from patients with tumor necrosis factor receptor-associated periodic syndrome (TRAPS), an auto-inflammatory disorder. mtROS levels can increase through the coupling of TLR1/2/4 signaling to mitochondrial Complex I via TNF Receptor-Associated Factor 6 (TRAF6) and the evolutionarily conserved signaling intermediate in the Toll pathway (ECSIT). Deletion of TRAF6 and ECSIT or overexpression of antioxidant enzymes in mitochondria leads to decreased mtROS and impaired bacterial killing. In the early stages of skin wound healing in mice, macrophages exhibit signs of a dysfunctional TCA cycle along with increased mtROS production [5]. Elevated mtROS influences HIF1α stabilization, converting macrophages into the M1 subtype, which promotes vascularization and inflammation during healing. Thus, mtROS signaling is essential for wound healing at both the single-cell and multicellular levels. However, it is crucial to recognize that excessive ROS production can damage tissues and result in nonhealing wounds in conditions such as diabetes. Accordingly, applying oxygen-releasing and ROS-scavenging hydrogels to diabetic wounds in mice may enhance healing by promoting angiogenesis, re-epithelialization, and reducing inflammation [5].
The intricate relationship between mitochondrial dynamics and wound healing underscores the critical role of mitochondrial function in cellular repair processes. Enhanced mitochondrial calcium uptake and the subsequent increase in mtROS are pivotal for effective wound healing, facilitating key processes such as actin ring formation, cell delamination, and angiogenesis. Studies across various model organisms, including C. elegans and Drosophila, have highlighted that mitochondrial fragmentation and mtROS production are essential for rapid and efficient wound closure. While these findings indicate a conserved mechanism of mtROS signaling in wound repair, the precise localization and regulation of this signaling at injury sites remain to be fully elucidated. Moreover, the dual role of mtROS as both a beneficial signaling molecule and a potential source of oxidative stress emphasizes the need for a balanced response to injury. Overproduction of ROS can lead to tissue damage and impaired healing, particularly in pathological conditions such as diabetes. Therefore, understanding the mechanistic pathways involved in mitochondrial dynamics and ROS signaling holds significant potential for developing therapeutic strategies aimed at enhancing wound healing and addressing chronic non-healing wounds. Future research should focus on the specific roles of mitochondrial dynamics in human tissues and explore the therapeutic implications of modulating mtROS levels to promote effective wound healing. In recent years, there has been a growing interest in mitochondrial therapy as an innovative approach to wound healing. This approach is based on the ability of mitochondria to regulate cellular metabolism, produce energy, and respond to oxidative stress. Future research in this area suggests that targeting the enhancement of mitochondrial function could accelerate the wound-healing process. Future studies may focus on the use of pharmacological agents that can enhance mitochondrial performance. For example, antioxidants and compounds that can increase ATP production may help improve the quality of wound healing. Additionally, exploring the role of nanoparticles and tissue engineering technologies in optimizing mitochondria and enhancing the wound-healing process will also be key research areas. Ultimately, with advancements in technology and a deeper understanding of the role of mitochondria in wound healing, we can expect the development of innovative approaches for treating skin injuries and improving the quality of life for patients. These approaches not only aid in enhancing the wound healing capability but can also be effective in reducing side effects and improving overall treatment outcomes.
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665-706. Available from: https://doi.org/10.1152/physrev.00067.2017
- Peña OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024:1-18. Available from: https://doi.org/10.1038/s41580-024-00715-1
- Ho J, Walsh C, Yue D, Dardik A, Cheema U. Current advancements and strategies in tissue engineering for wound healing: a comprehensive review. Adv Wound Care (New Rochelle). 2017;6(6):191-209. Available from: https://doi.org/10.1089/wound.2016.0723
- Monzel AS, Enríquez JA, Picard M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab. 2023;5(4):546-62. Available from: https://doi.org/10.1038/s42255-023-00783-1
- Willenborg S, Sanin DE, Jais A, Ding X, Ulas T, Nüchel J, et al. Mitochondrial metabolism coordinates stage-specific repair processes in macrophages during wound healing. Cell Metab. 2021;33(12):2398-414.e9. Available from: https://doi.org/10.1016/j.cmet.2021.10.004
- Qing C. The molecular biology in wound healing & non-healing wound. Chin J Traumatol. 2017;20(04):189-93. Available from: https://doi.org/10.1016/j.cjtee.2017.06.001
- Yao WD, Zhou JN, Tang C, Zhang JL, Chen ZY, Li Y, et al. Hydrogel Microneedle Patches Loaded with Stem Cell Mitochondria-Enriched Microvesicles Boost the Chronic Wound Healing. ACS Nano. 2024;18(39):26733-50. Available from: https://doi.org/10.1021/acsnano.4c06921
- Licini C, Morroni G, Lucarini G, Vitto VAM, Orlando F, Missiroli S, et al. ER-mitochondria association negatively affects wound healing by regulating NLRP3 activation. Cell Death Dis. 2024;15(6):407. Available from: https://doi.org/10.1038/s41419-024-06765-9
- Taner OF, Ulger O, Ersahin S, Baser NT, Genc O, Kubat GB. Effects of mitochondrial transplantation on chronic pressure wound healing in a human patient. Cytotherapy. 2024;26(6):579-85. Available from: https://doi.org/10.1016/j.jcyt.2024.02.027
- Wang M, Yang D, Li L, Wu P, Sun Y, Zhang X, et al. A Dual Role of Mesenchymal Stem Cell-Derived Small Extracellular Vesicles on TRPC6 Protein and Mitochondria to Promote Diabetic Wound Healing. ACS Nano. 2024;18(6):4871-85. Available from: https://doi.org/10.1021/acsnano.3c09814
- Benard G, Bellance N, Jose C, Melser S, Nouette-Gaulain K, Rossignol R. Multi-site control and regulation of mitochondrial energy production. Biochim Biophys Acta Bioenerg. 2010;1797(6-7):698-709. Available from: https://doi.org/10.1016/j.bbabio.2010.02.030
- Bansal R, Torres M, Hunt M, Wang N, Chatzopoulou M, Manchanda M, et al. Role of the mitochondrial protein cyclophilin D in skin wound healing and collagen secretion. JCI Insight. 2024;9(9). Available from: https://doi.org/10.1172/jci.insight.169213
- Qi X, Liu C, Si J, Yin B, Huang J, Wang X, et al. A bioenergetically‐active poly(glycerol sebacate)‐based multiblock hydrogel improved diabetic wound healing through revitalizing mitochondrial metabolism. Cell Prolif. 2024:e13613. Available from: https://doi.org/10.1111/cpr.13613
- Wang H, Gong J, Chen W, Sun Q, Zhang T, Lin Y, et al. Tetrahedral framework nucleic acids’ role in facilitating chronic diabetic wound repair via the endoplasmic reticulum-mitochondrial pathway. Nano Today. 2024;56:102252. Available from: https://doi.org/10.1016/j.nantod.2024.102252
- Zhang S, Peng B, Qi Y, Xu C, Wang Y, Xian T, et al. Dual response Hst1@ CBTC hydrogel promoting diabetic wounds healing by improving mitochondrial autophagy and inhibiting ferroptosis via Nrf2/HO-1. Chem Eng J. 2024;492:152358. https://doi.org/10.1016/j.cej.2024.152358
- Bai L, Wu L, Zhang C, Liu Z, Ma L, Ni J, et al. Replenishment of mitochondrial Na+ and H+ by ionophores potentiates cutaneous wound healing in diabetes. Mater Today Bio. 2024;26:101056. https://doi.org/10.1016/j.mtbio.2024.101056
- Gonzalez ACdO, Costa TF, Andrade ZdA, Medrado ARAP. Wound healing-A literature review. Anais Bras Dermatol. 2016;91(5):614-20. Available from: https://doi.org/10.1016/j.mtbio.2024.101056
- Visha M, Karunagaran M. A review on wound healing. Int J Clin Correl. 2019;3(2):50. https://www.editorialmanager.in/index.php/ijcpc/article/view/404
- Mercandetti M, Cohen A. Wound healing and repair. Emedicine. 2017;14(1):12-20. Available from: https://doi.org/10.1159/000339613
- Strodtbeck F. Physiology of wound healing. Newborn Infant Nurs Rev. 2001;1(1):43-52. Available from: https://doi.org/10.1053/nbin.2001.23176
- Ren H, Zhao F, Zhang Q, Huang X, Wang Z. Autophagy and skin wound healing. Burns Trauma. 2022;10:tkac003. Available from: https://doi.org/10.1093/burnst/tkac003
- Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34:599-610. Available from: https://doi.org/10.1007/s12325-017-0478-y
- Xu Z, Han S, Gu Z, Wu J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv Healthc Mater. 2020;9(5):1901502. Available from: https://doi.org/10.1002/adhm.201901502
- Atkin L, Bućko Z, Montero EC, Cutting K, Moffatt C, Probst A, et al. Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care. 2019;28(Sup3a):S1-S50. Available from: https://doi.org/10.12968/jowc.2019.28.sup3a.s1
- Theocharidis G, Thomas BE, Sarkar D, Mumme HL, Pilcher WJ, Dwivedi B, et al. Single-cell transcriptomic landscape of diabetic foot ulcers. Nat Commun. 2022;13(1):181. Available from: https://doi.org/10.1038/s41467-021-27801-8
- Vercellino I, Sazanov LA. The assembly, regulation and function of the mitochondrial respiratory chain. Nat Rev Mol Cell Biol. 2022;23(2):141-61. Available from: https://doi.org/10.1038/s41580-021-00415-0
- Sies H, Mailloux RJ, Jakob U. Fundamentals of redox regulation in biology. Nat Rev Mol Cell Biol. 2024:1-19. Available from: https://doi.org/10.1038/s41580-024-00730-2
- Xu S, Li S, Bjorklund M, Xu S. Mitochondrial fragmentation and ROS signaling in wound response and repair. Cell Regen. 2022;11(1):38. Available from: https://doi.org/10.1186/s13619-022-00141-8
- Chen Y, Culetto E, Legouis R. The strange case of Drp1 in autophagy: Jekyll and Hyde? Bioessays. 2022;44(4):2100271. Available from: https://doi.org/10.1002/bies.202100271
- Chen M, Chen Z, Wang Y, Tan Z, Zhu C, Li Y, et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy. 2016;12(4):689-702. Available from: https://doi.org/10.1080/15548627.2016.1151580
- Nemani N, Carvalho E, Tomar D, Dong Z, Ketschek A, Breves SL, et al. MIRO-1 determines mitochondrial shape transition upon GPCR activation and Ca2+ stress. Cell Rep. 2018;23(4):1005-19. Available from: https://doi.org/10.1016/j.celrep.2018.03.098
- Levoux J, Prola A, Lafuste P, Gervais M, Chevallier N, Koumaiha Z, et al. Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab. 2021;33(2):283-99.e9. Available from: https://doi.org/10.1016/j.cmet.2020.12.006
- Kowaltowski AJ, Menezes-Filho SL, Assali EA, Gonçalves IG, Cabral-Costa JV, Abreu P, et al. Mitochondrial morphology regulates organellar Ca2+ uptake and changes cellular Ca2+ homeostasis. FASEB J. 2019;33(12):13176. Available from: https://doi.org/10.1096/fj.201901136r
- Bravo-Sagua R, Parra V, López-Crisosto C, Díaz P, Quest A, Lavandero S. Calcium transport and signaling in mitochondria. Compr Physiol. 2017;7(2):623-34. Available from: https://doi.org/10.1002/cphy.c160013
- Romero-Garcia S, Prado-Garcia H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer. Int J Oncol. 2019;54(4):1155-67. Available from: https://doi.org/10.3892/ijo.2019.4696
- Parnis J, Montana V, Delgado-Martinez I, Matyash V, Parpura V, Kettenmann H, et al. Mitochondrial exchanger NCLX plays a major role in the intracellular Ca2+ signaling, gliotransmission, and proliferation of astrocytes. J Neurosci. 2013;33(17):7206-19. Available from: https://doi.org/10.1523/jneurosci.5721-12.2013
- Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat Rev Mol Cell Biol. 2013;14(4):249-62. Available from: https://doi.org/10.1038/nrm3541
- Antunes M, Pereira T, Cordeiro JV, Almeida L, Jacinto A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J Cell Biol. 2013;202(2):365-79. Available from: https://doi.org/10.1083/jcb.201211039
- Horn A, Raavicharla S, Shah S, Cox D, Jaiswal JK. Mitochondrial fragmentation enables localized signaling required for cell repair. J Cell Biol. 2020;219(5):e201909154. Available from: https://doi.org/10.1083/jcb.201909154
- Fu H, Zhou H, Yu X, Xu J, Zhou J, Meng X, et al. Wounding triggers MIRO-1-dependent mitochondrial fragmentation that accelerates epidermal wound closure through oxidative signaling. Nat Commun. 2020;11(1):1050. Available from: https://doi.org/10.1038/s41467-020-14885-x
- Ponte S, Carvalho L, Gagliardi M, Campos I, Oliveira PJ, Jacinto A. Drp1-mediated mitochondrial fission regulates calcium and F-actin dynamics during wound healing. Biol Open. 2020;9(5):bio048629. Available from: https://doi.org/10.1242/bio.048629
- Hunt M, Torres M, Bachar-Wikström E, Wikström JD. Multifaceted roles of mitochondria in wound healing and chronic wound pathogenesis. Front Cell Dev Biol. 2023;11:1252318. Available from: https://doi.org/10.3389/fcell.2023.1252318
- Chen H, Chan DC. Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab. 2017;26(1):39-48. Available from: https://doi.org/10.1016/j.cmet.2017.05.016
- Brandt T, Cavellini L, Kühlbrandt W, Cohen MM. A mitofusin-dependent docking ring complex triggers mitochondrial fusion in vitro. Elife. 2016;5:e14618. Available from: https://doi.org/10.7554/elife.14618
- Zomer HD, Trentin AG. Skin wound healing in humans and mice: Challenges in translational research. J Dermatol Sci. 2018;90(1):3-12. Available from: https://doi.org/10.1016/j.jdermsci.2017.12.009
- Mellem D, Sattler M, Pagel-Wolff S, Jaspers S, Wenck H, Rübhausen MA, et al. Fragmentation of the mitochondrial network in skin in vivo. PLoS One. 2017;12(6):e0174469. Available from: https://doi.org/10.1371/journal.pone.0174469
- Le Moal E, Pialoux V, Juban G, Groussard C, Zouhal H, Chazaud B, et al. Redox control of skeletal muscle regeneration. Antioxid Redox Signal. 2017;27(5):276-310. Available from: https://doi.org/10.1089/ars.2016.6782
- Ma Y, Xie J, Wijaya CS, Xu S. From wound response to repair–lessons from C. elegans. Cell Regen. 2021;10:1-10. Available from: https://doi.org/10.1186/s13619-020-00067-z
- Zhao H, Chen J, Chai J, Zhang Y, Yu C, Pan Z, et al. Cytochrome P450 (CYP) epoxygenases as potential targets in the management of impaired diabetic wound healing. Lab Invest. 2017;97(7):782-91. Available from: https://doi.org/10.1038/labinvest.2017.21